03 May Microfluidic model shows α‑Synuclein spreads backward along axons
Lewy bodies -intracellular aggregates rich in α‑Synuclein (αSyn)- appear in a stereotyped pattern as Parkinson’s disease (PD) progresses, implying that mis‑folded αSyn travels from neuron to neuron. Animal studies and human autopsy work strongly hint that “up‑stream” axons carry the seeds backward toward their cell bodies, yet in‑vitro evidence has been mixed. A key obstacle has been building culture systems that keep axons growing in only one direction for the many weeks human iPSC‑derived neurons need to mature.
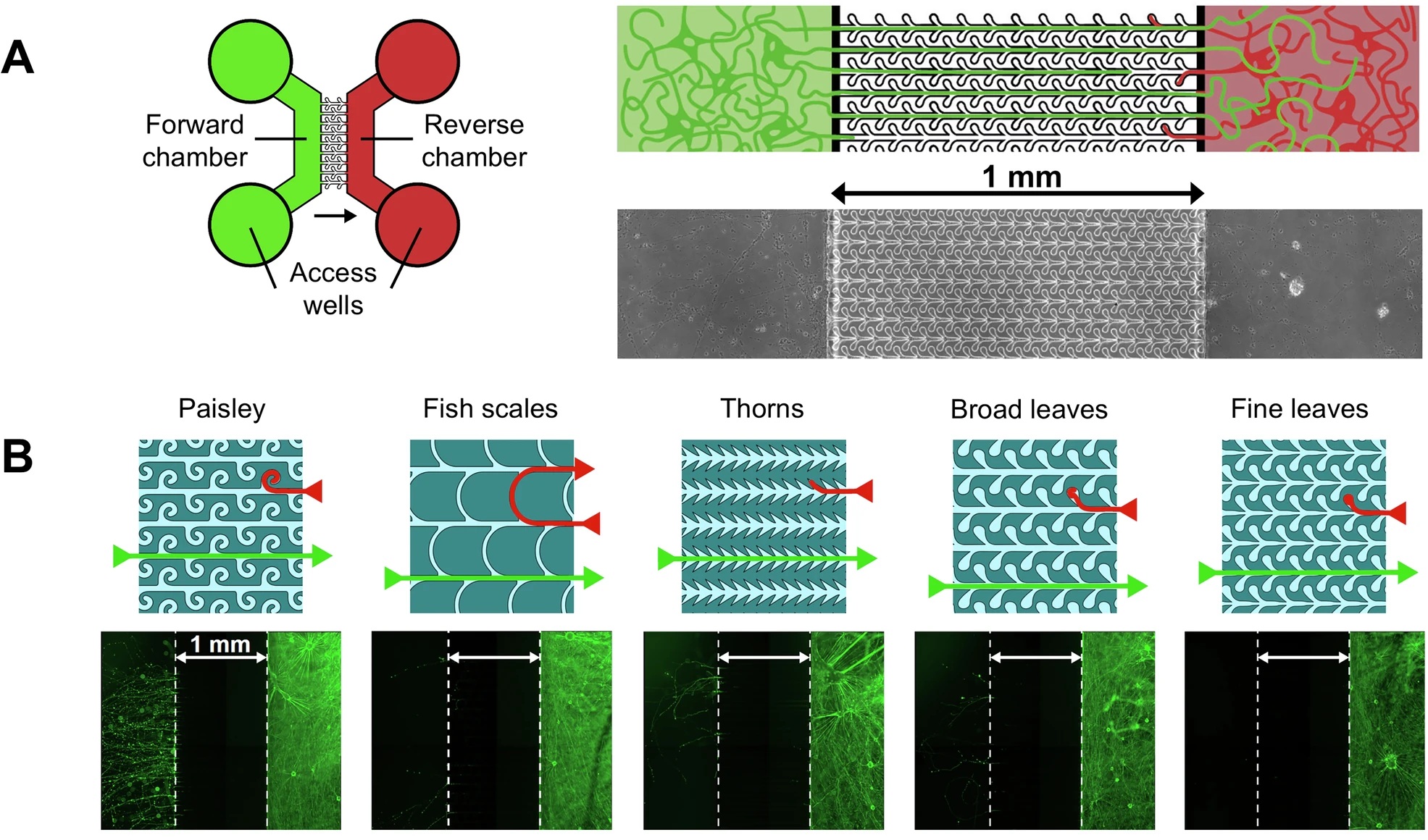
A new study reports a microfluidic chip that holds human induced‑pluripotent‑stem‑cell‑derived mid‑brain dopaminergic neurons in two fluid‑isolated chambers linked by 1 mm‑long microchannels. The channels are patterned with “fine‑leaf” dead‑ends that let axons grow forward but trap any that try to reverse course, so unidirectional connections remain intact for more than three months—long enough for the neurons to express adult markers and, crucially, long enough to watch pathological α‑Synuclein move.
“To elucidate how αSyn pathology propagates between brain areas, we developed a novel in vitro microfluidic platform to study the intracellular transport of preformed fibrils and the induction and spread of αSyn aggregates. “, the authors explained.
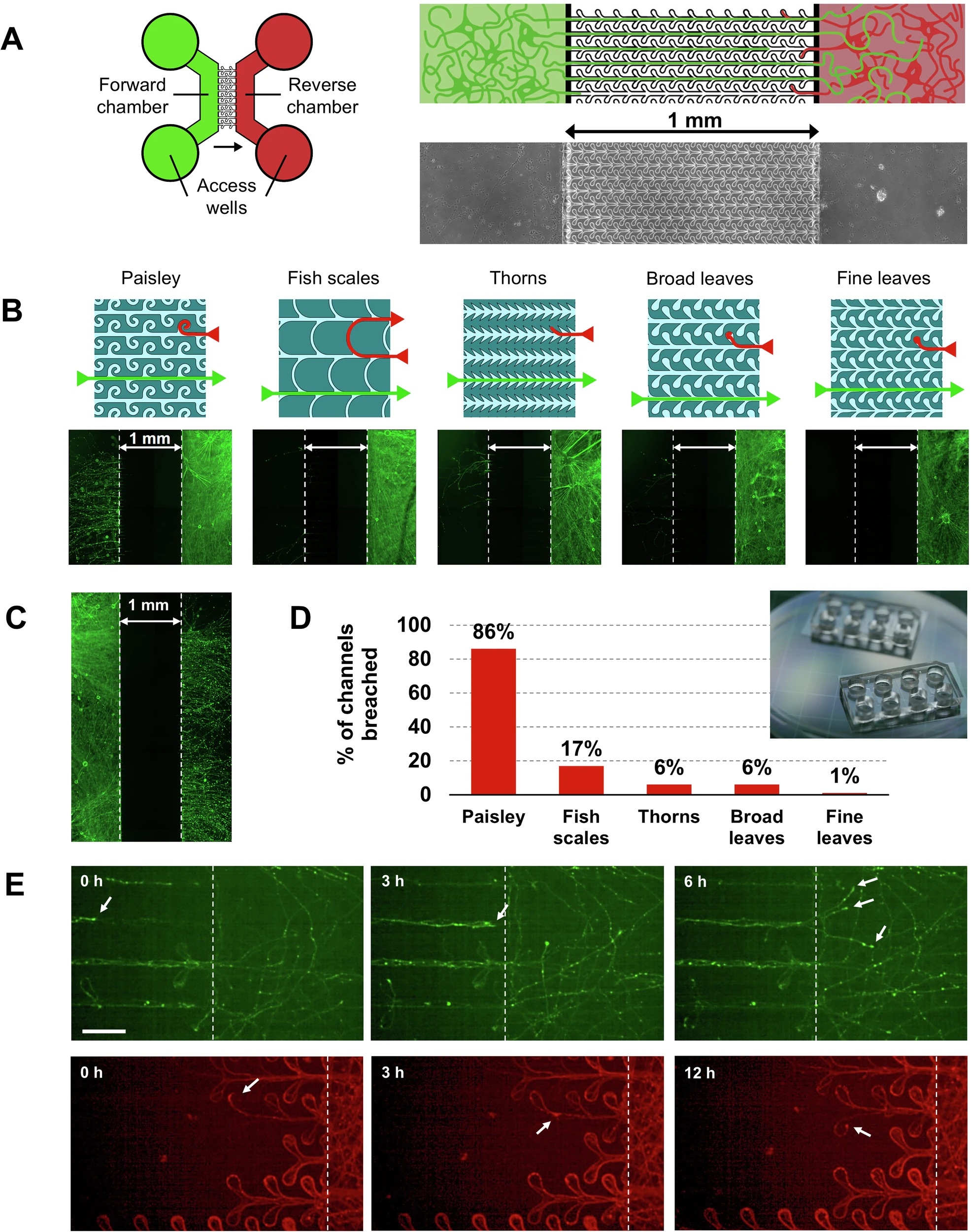
“A Schematic of the device. Left: The design of the microfluidics device consisting of two chambers that are connected by the patterned microchannels such that axons can only reach the other chamber from left to right. The circles are the wells that give access to the chambers and are used for plating cells, refreshing the medium and seeding PFFs. The green side is the forward side and the red the reverse side. The arrow indicates the direction in which neurites are allowed to grow unhindered. Right: Close-up of the schematic to show the microchannels and underneath an image of the actual device. B Schematics of the 5 microchannel patterns and underneath fluorescence microscopy image of devices stained with βIII-tubulin showing the effectiveness of the unidirectional design. mDA cells were plated in the Reverse chamber only. The ‘fine leaves’ design was most effective in preventing axons from crossing to the Forward chamber. Note that the neurites within microchannels need a longer exposure-time and are therefore not visible in this example. C Axons from neurons cultured in the forward chamber were not hindered in reaching the reverse chamber, here shown for the ‘fine leaves’ design. D Graph showing the varying extent to which the microchannels of different design were breached. E Mouse hippocampal neurons expressing EGFP were cultured in the forward chamber while neurons expressing m-cherry were simultaneously cultured in the reverse chamber (or vice versa). Images are shown from a time-lapse experiment. Top: temporal progression of a microfluidic culture showing forward-originating axons reaching the reverse chamber. Arrows indicate example growth cones. Bottom: temporal progression showing axons originating in the reverse chamber remaining trapped in the ‘dead-end’ leaves of the microfluidic pattern or reversing growth direction and returning towards the originating chamber. The scale bar is 50 µm.” Reproduced from Vroman, R., de Lichtervelde, L., Singh Dolt, K. et al. A high-fidelity microfluidic platform reveals retrograde propagation as the main mechanism of α-Synuclein spread in human neurons. npj Parkinsons Dis. 11, 80 (2025). under a CC BY 4.0 Attribution 4.0 International license
The team seeded Alexa‑568–labelled α‑Synuclein pre‑formed fibrils (PFFs) into either the “forward” or “reverse” chamber of the microfluidic device while maintaining a slight hydrostatic pressure offset to block passive diffusion. High‑resolution confocal imaging showed that PFF puncta entered axons within minutes, but only those added to the reverse microfluidic chamber crossed the 1 mm barrier efficiently. Small aggregates travelled retrogradely at roughly 1.1 µm s⁻¹, appearing in the forward chamber’s axons within hours and in its cell bodies by one day. In contrast, anterograde crossings were rare and slow; none were detected in the first 24 h and only sporadic events emerged after five days.
Three weeks after the initial pulse, neurons on the retrograde side were laden with Lewy‑body‑like inclusions: pSer129‑α‑Synuclein that co‑localised with the autophagy adaptor p62 and with MAP2‑positive dendrites. Cultures seeded in the forward chamber—therefore relying on anterograde transport—showed minimal pathology on the opposite side. Because the two fluid compartments stayed isolated throughout, the authors could rule out simple extracellular spread; pathology followed the same direction as the moving PFFs, strengthening the case that intracellular retrograde transport drives propagation.
Taken together, the work provides a long‑term, high‑fidelity human model of α‑Synuclein spread and shows that, under these controlled microfluidic conditions, the fibrils ride axonal highways predominantly toward the soma. The device should be a valuable testbed for compounds that aim to slow or block retrograde trafficking, for genetic screens targeting axonal transport machinery, or for probing whether other aggregation‑prone proteins—tau, TDP‑43, huntingtin—obey the same directional rules in human neurons.
“Combining these co-culture models with lab-on-chip methodologies will result in increasingly more refined models with in vivo-like physiological conditions, providing a useful tool to assess the interaction between αSyn pathology propagation and other mechanisms at play in PD pathogenesis, as well as a general tool for studying other proteinopathies and screening of potential disease-modifying treatments.“, the authors concluded
Figures are reproduced from Vroman, R., de Lichtervelde, L., Singh Dolt, K. et al. A high-fidelity microfluidic platform reveals retrograde propagation as the main mechanism of α-Synuclein spread in human neurons. npj Parkinsons Dis. 11, 80 (2025). https://doi.org/10.1038/s41531-025-00936-x under a CC BY 4.0 Attribution 4.0 International license.
Read the original article: A high-fidelity microfluidic platform reveals retrograde propagation as the main mechanism of α-Synuclein spread in human neurons
For more insights into the world of microfluidics and its burgeoning applications in biomedical research, stay tuned to our blog and explore the limitless possibilities that this technology unfolds. If you need high quality microfluidics chip for your experiments, do not hesitate to contact us.


