09 May Microfluidic Human Neurovascular‑Unit Chip Illuminates How HSV‑1 Triggers Brain Barrier Failure
Herpes simplex virus type 1 (HSV‑1) remains the leading cause of sporadic viral encephalitis, killing a third of patients even when acyclovir is given promptly and leaving most survivors with lasting neurological damage. Why the virus wreaks such selective havoc inside the human brain has long puzzled clinicians, because classic animal or two‑dimensional cell models cannot fully mirror the tightly knit partnership between brain vessels and parenchyma. A recent article published in Nature Communications employed microfluidics technology and built a miniaturised, living copy of the human neurovascular unit (NVU) on a microfluidic chip.
“In this work, we microengineered a new in vitro 3D NVU model that allows to reconstitute the BBB interface and NVU structure by co-culturing brain endothelial cells, microglia, astrocytes and neurons in a biomimetic brain microenvironment. This model can not only emulate the features of NVU in normal condition but also enables to monitor the multi-stage intercellular interactions in pathological condition dynamically, such as the responses of peripheral immune cells and microglia. “, the authors explained.
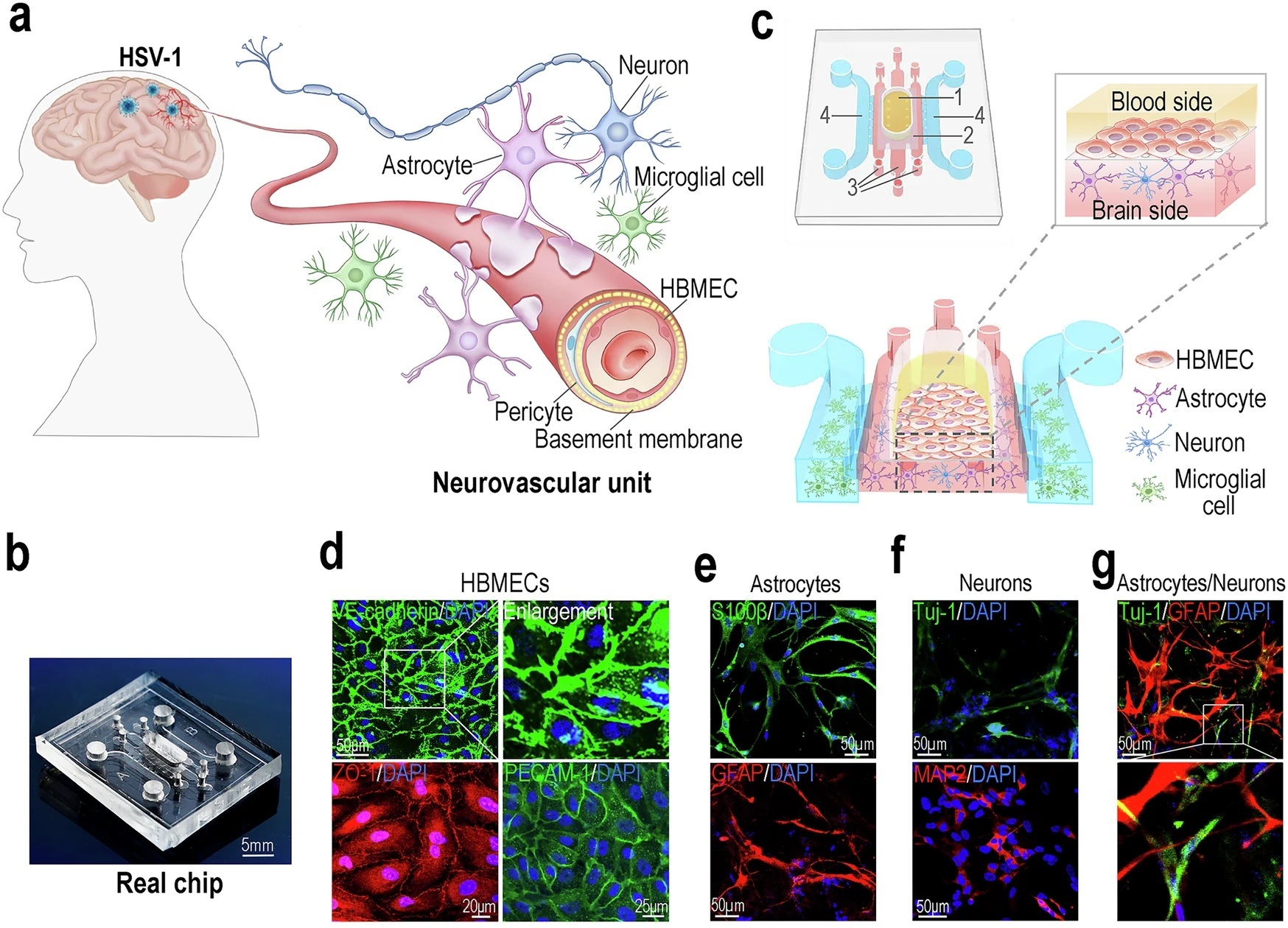
They layered primary brain micro‑vascular endothelial cells above a porous membrane in the microfluidic device, then embedded human astrocytes, neurons and microglia in three‑dimensional Matrigel channels below, recreating an in‑vitro blood–brain barrier that resists 4 kDa dextran leakage and shows aquaporin‑4‑positive astrocytic end‑feet—hallmarks of an intact NVU.
When HSV‑1 was delivered exclusively to the “brain side”, the virus bloomed in glia and neurons but spared the endothelial frontier. Damage nevertheless rippled back across the barrier: VE‑cadherin vanished from endothelial junctions, dextran permeability rose, and lactate‑dehydrogenase levels climbed in both compartments, capturing the vascular dysfunction reported in patient autopsy series. Dying neurons released the inflammatory signals IL‑1β, IL‑6, TNF‑α, CXCL1 and IFN‑β, while RFP‑labelled microglia streamed through trapezoidal migration channels toward the infected core, mirroring the innate immune infiltration seen in vivo.
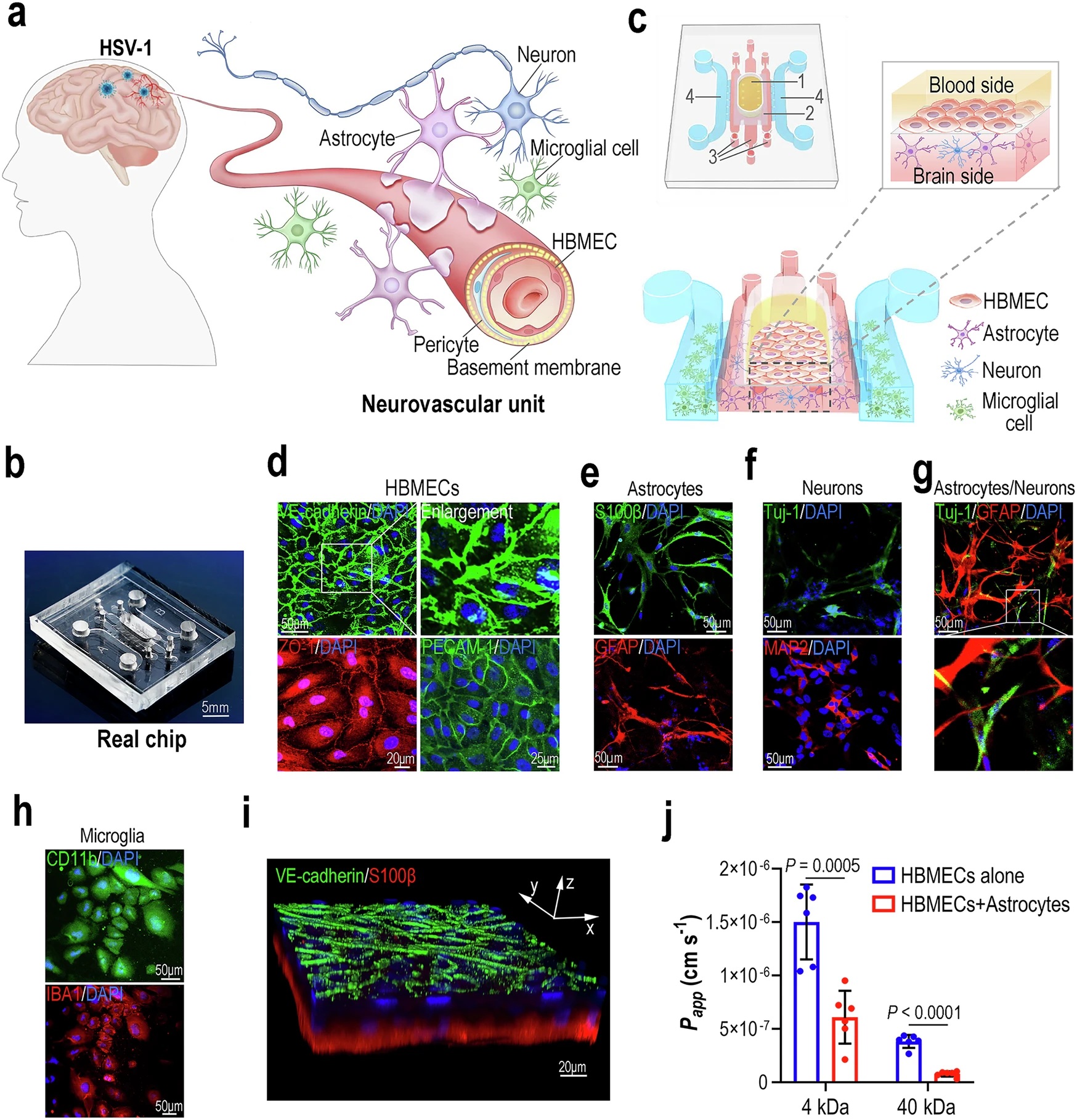
“a Schematic diagram of the human neurovascular unit. b Image of a real chip. c 3D schematic diagram showing cell culture on the NVU chip. The yellow area indicates the HBMECs culture compartment of the blood side (1). The dark gray area indicates the porous PCTE membrane between the blood side and brain side (2). The red area indicates the astrocytes/neurons culture compartment of the brain side (3). The blue area indicates the microglia culture compartment of the brain side (4). d 3 days after seeding cells on the chip, confocal micrographs showing the HBMECs on the chip, immunostained for VE-cadherin, ZO-1 and PECAM-1. e Confocal micrographs showing the astrocytes on the chip, immunostained for S100β and GFAP. f Confocal micrographs showing the neurons on the chip, stained for Tuj-1 and MAP2. g A confocal micrograph showing the co-culture of astrocytes (GFAP) and neurons (Tuj-1) in Matrigel. h Confocal micrographs showing the microglia on the chip, immunostained for CD11b and IBA1. i A 3D configuration image showing BBB interface formed by HBMECs (VE-cadherin) and astrocytes (S100β) after co-culture for 3 days. d–i Representative images were from 3 biological replicates. All experiments were repeated at least 3 times. j FITC-dextran permeability assays for BBB formed by HBMECs alone, or HBMECs and astrocytes, after culture for 3 days (n = 6 for biological replicates; n = 3 for technical replicates). Data are presented as the mean ± SD and were analyzed using an unpaired two-sided Student’s t-test.” Reproduced from Zhang, M., Wang, P., Wu, Y. et al. A microengineered 3D human neurovascular unit model to probe the neuropathogenesis of herpes simplex encephalitis. Nat Commun 16, 3701 (2025). under a CC BY 4.0 Attribution 4.0 International license
Transcriptome analysis of the microfluidic chip revealed a broad chemotactic and cytokine response, but also a surprising hostage situation inside glia: HSV‑1 blocked autophagic flux. Pharmacological activators that reopen autophagy not only curtailed viral replication but also restored barrier tightness and cell viability, hinting that reinforcing a neuron’s own recycling machinery could complement direct antivirals in the clinic. The microfluidic chip even reproduced the link between HSV‑1 and Alzheimer pathology—Aβ42, but not Aβ40, accumulated around infected cells by day 4, offering a microfluidic platform to test how viral assaults may seed neurodegeneration.
Altogether, this microfluidic human 3D NVU‑on‑a‑chip provides a window onto the step‑by‑step neuropathogenesis of herpes simplex encephalitis: selective glial infection, microglial mobilisation, cytokine storm, barrier collapse and secondary endothelial damage. Because every cell type sits where it does in the living brain, researchers can now record how future antiviral, anti‑inflammatory or pro‑autophagy compounds modify each stage in real time, without the species gap that hampers mouse work.
“Taken together, we established a new 3D NVU model that allows to recapitulate the features of NVU in normal and pathological condition of HSV-1 infection in vitro. The novelty of this work lies in its ability to mimic the physiologically relevant microenvironment of NVU and the host immune responses to viral infection in a human-organ context.“, the authors concluded
Figures are reproduced from Zhang, M., Wang, P., Wu, Y. et al. A microengineered 3D human neurovascular unit model to probe the neuropathogenesis of herpes simplex encephalitis. Nat Commun 16, 3701 (2025). https://doi.org/10.1038/s41467-025-59042-4 under a CC BY 4.0 Attribution 4.0 International license.
Read the original article: A microengineered 3D human neurovascular unit model to probe the neuropathogenesis of herpes simplex encephalitis
For more insights into the world of microfluidics and its burgeoning applications in biomedical research, stay tuned to our blog and explore the limitless possibilities that this technology unfolds. If you need high quality microfluidics chip for your experiments, do not hesitate to contact us.


