12 Jun Ultrafast and Scalable CAR-T Manufacturing Using Microfluidics
Chimeric antigen receptor T-cell (CAR-T) therapy has reshaped the treatment landscape for hematologic cancers, but the process of manufacturing CAR-T cells remains a major bottleneck. Traditional protocols might stretch over 9 days and risk shifting T-cell populations toward terminally differentiated phenotypes, depleting the naive-like T-cells (Tnlp) essential for long-lasting therapeutic responses. This extended culture period is not only time-consuming but may also limit the quality and efficacy of the final CAR-T product.
To address this challenge, researchers developed a microfluidic device (MFD) that integrates T-cell activation and lentiviral transduction into a single step, shortening the entire CAR-T manufacturing process to under 24 hours. By designing this microfluidic chip, the research team aimed at a protocol that not only accelerate production but also enhances the retention of the Tnlp phenotype in the final CAR-T product—critical for effective and durable cancer treatment.
“CAR-T manufacturing remains a major bottleneck of this treatment modality; in standard cases, it takes up to two weeks, resulting in a phenotypic shift toward terminally differentiated T-cells and a significant depletion of T-cells with naive-like phenotype (Tnlp), crucial for sustained clinical efficacy. Leveraging the current progress in microfluidic technologies, we develop and optimize a microfluidic device (MFD) for CAR-T cell production via an ultrafast protocol that integrates T-cell activation and lentiviral transduction in a single step within 24 hours. “, the authors explained.
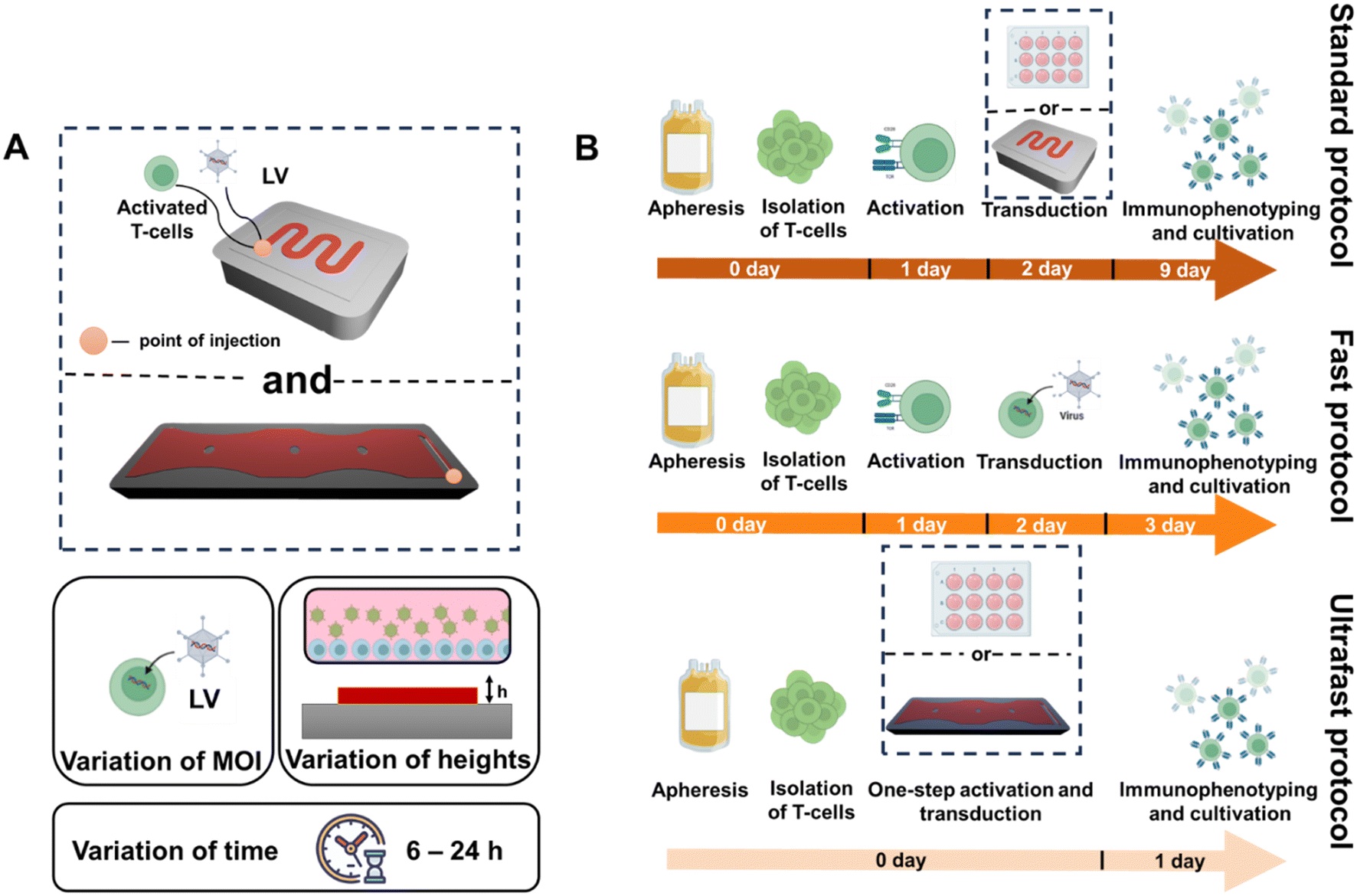
The microfabrication of the microfluidic chip was conducted using soft lithography and polydimethylsiloxane (PDMS), with optimized geometries to facilitate high-efficiency transduction. Researchers varied the microfluidic channel heights, incubation times, and multiplicities of infection (MOIs) to fine-tune the microfluidic system. They ultimately selected a 150 μm microchannel height for its balance between fluid handling ease and viral exposure. T-cells were simultaneously activated using biodegradable nanoparticles and transduced with third-generation lentiviral vectors encoding anti-CD19 CAR constructs. Flow cytometry was used to assess both CAR expression and T-cell phenotype at multiple time points.

“(A) Schematic illustration of the used MFD and performed optimization steps, which include variation of MOI, channel heights, and incubation time. (B) Schematic illustration of ultrafast, fast, and standard protocols.” Reproduced fromV. Markelov, K. V. Arabuli, I. Gaponenko, V. Sergeev, A. Shakirova, K. V. Lepik, A. D. Kulagin and M. V. Zyuzin, Lab Chip, 2025, 25, 3005 DOI: 10.1039/D5LC00139K with permission from Royal Society of Chemistry.
Results showed that transduction efficiency reached 27% in the microfluidic chip at MOI 3, outperforming 48-well (17%) and 6-well (8%) plate controls. More importantly, the ultrafast protocol preserved significantly higher percentages of naive-like CD3+, CD4+, and CD8+ T-cells compared to both fast (72-hour) and standard (9-day) manufacturing protocols. Specifically, CD3+ Tnlp levels were 18.07% in the MFD compared to just 3.97% after 9 days in standard protocols. These trends were echoed in CD4+ (11.07% vs. 3.56%) and CD8+ (29.2% vs. 4.18%) Tnlp levels.
By minimizing culture time, the microfluidic platform prevents the differentiation of Tnlp into more mature, less proliferative subsets. This feature could be vital for improving CAR-T cell persistence and potency in patients. The platform also demonstrated consistent cell viability and proliferation rates, suggesting it can scale effectively for clinical use.
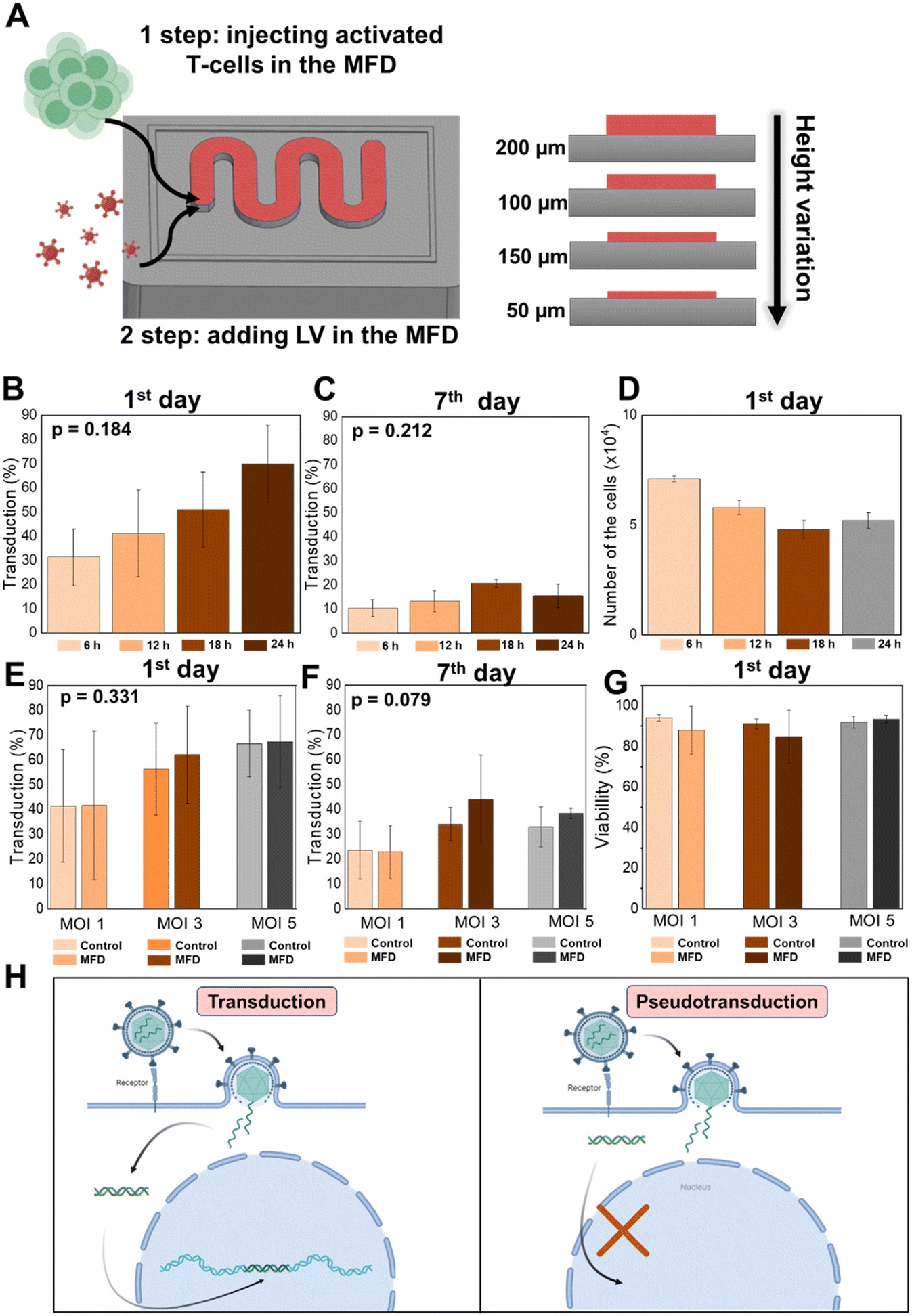
“Optimization of CAR T-cell production using MFD. (A) Schematic illustration of CAR T-cell production in our MFD. (B) Transduction efficiency of T-cells at fixed MOI 3 on the 1st day (6, 12, 18, 24 h time points) in an MFD. (C) Transduction efficiency of T-cells at fixed MOI 3 on the 7th day (6, 12, 18, 24 h time points) in an MFD. D. T-cells yield on the 1st day (6, 12, 18, 24 h time points) in an MFD. E. Transduction efficiency of T-cells at different MOIs (1, 3, 5) on the 1st day (6, 12, 18, 24 h time points) in an MFD. F. Transduction efficiency of T-cells at different MOIs (1, 3, 5) on the 7th day (6, 12, 18, 24 h time points) in an MFD. G. T-cell viability at different MOIs (1, 3, 5) on the 1st day (6, 12, 18, 24 h time points) in an MFD. H. Schematic illustration of the transduction and pseudotransduction processes.” Reproduced fromV. Markelov, K. V. Arabuli, I. Gaponenko, V. Sergeev, A. Shakirova, K. V. Lepik, A. D. Kulagin and M. V. Zyuzin, Lab Chip, 2025, 25, 3005 DOI: 10.1039/D5LC00139K with permission from Royal Society of Chemistry.
In conclusion, this study showcases a robust microfluidic-based strategy for ultrafast CAR-T cell manufacturing. The reduced timeline, combined with superior preservation of critical T-cell phenotypes, supports its potential as a viable platform for efficient and accessible CAR-T therapy production.
“Our results highlight MFDs as a scalable platform to streamline CAR-T manufacturing, with the potential to improve clinical accessibility and outcomes by reducing the production time while preserving essential T-cell phenotypes“, the authors concluded
Figures are reproduced fromV. Markelov, K. V. Arabuli, I. Gaponenko, V. Sergeev, A. Shakirova, K. V. Lepik, A. D. Kulagin and M. V. Zyuzin, Lab Chip, 2025, 25, 3005 DOI: 10.1039/D5LC00139K with permission from Royal Society of Chemistry.
Read the original article: Scalable and ultrafast CAR-T cell production using microfluidics
For more insights into the world of microfluidics and its burgeoning applications in biomedical research, stay tuned to our blog and explore the limitless possibilities that this technology unfolds. If you need high quality microfluidics chip for your experiments, do not hesitate to contact us.


