07 Oct Microfluidic Mapping of the Molecular Aging of Protein Condensates
Biomolecular condensates, membraneless structures formed through phase separation of proteins and nucleic acids, play a central role in regulating key cellular processes. Over time, however, some of these condensates lose their liquid-like nature and begin to solidify, a transformation that has been linked to neurodegenerative conditions such as amyotrophic lateral sclerosis (ALS). Despite their biological importance, understanding how these phase-separated assemblies evolve over time has been challenging. The main obstacle lies in the difficulty of studying condensates without disrupting their delicate molecular organization, making it hard to capture how structural and mechanical properties change during aging.
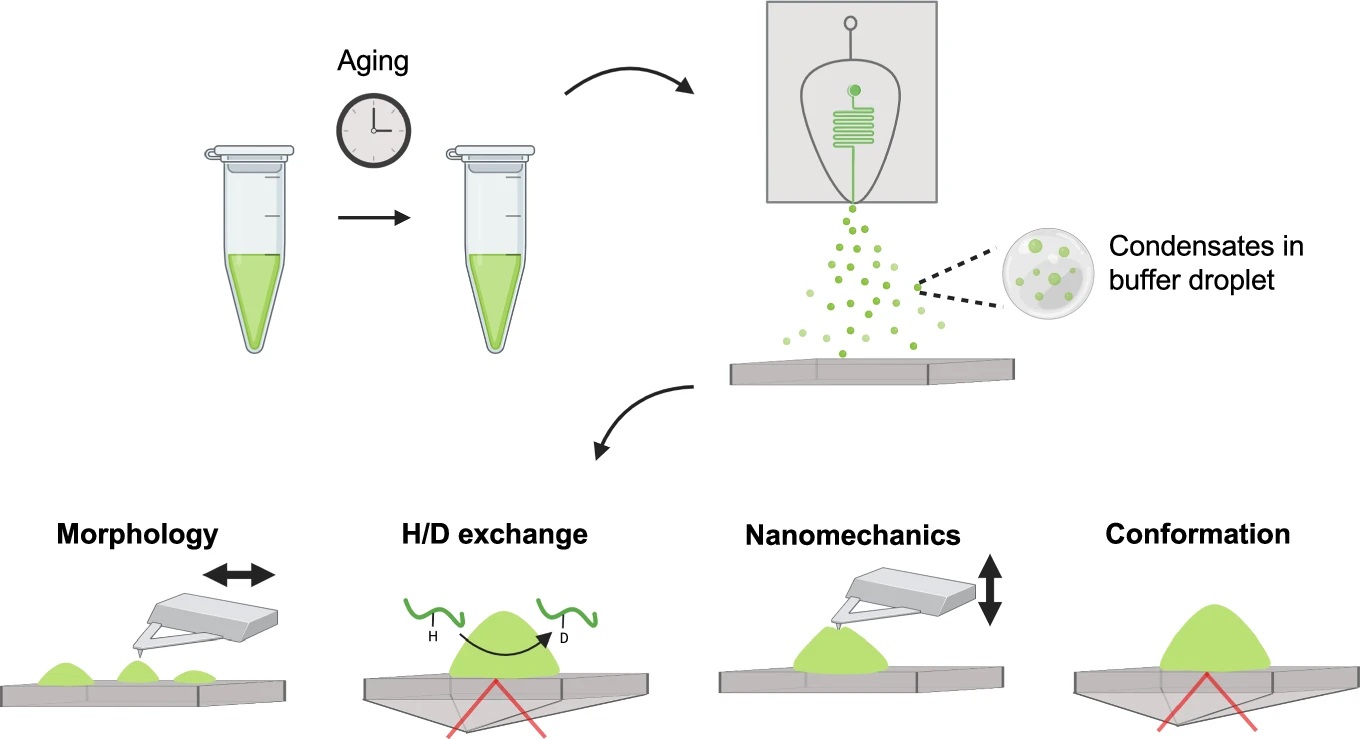
To address this challenge, researchers from the University of Cambridge and Wageningen University developed a combined experimental approach that bridges microfluidic chips, atomic force microscopy (AFM), and infrared (FTIR) spectroscopy. Using this integrated platform, they examined condensates formed by the FUS protein, an RNA-binding protein implicated in ALS, and followed their evolution over time at nanometer resolution. Their key innovation lies in a microfluidic device for spray deposition technique that allows the transfer of condensates from solution to a surface without altering their native structure. By generating picoliter droplets that dry within milliseconds, this method preserves the hydration and conformational integrity of delicate protein assemblies, enabling high-resolution mapping of their structural and mechanical features.
“Here, to address this problem, we combine a microfluidic sample deposition technique that preserves the solution properties of condensates on surfaces with a nanometre-resolution spatial mapping method to characterise the time-dependent material properties of condensates of the fused in sarcoma (FUS) protein.”, the authors explained.

“Overview of the experiments employed in this study. Condensates were deposited using a microfluidic spray device, which enables their characterisation via surface-based techniques. Four properties of condensates were probed as a function of ageing: (1) 3D morphology via AFM, (2) hydrogen-deuterium exchange rates via IR spectroscopy, (3) material properties via AFM nanomechanical mapping, and (4) chemical and structural properties via IR spectroscopy. This combined approach allowed us to generate a detailed model of how phases emerge in space and time within FUS condensates, and how these phases correlate with conformational changes at the polypeptide level.” Reproduced from Miller, A., Toprakcioglu, Z., Qamar, S. et al. Nanoscale profiling of evolving intermolecular interactions in ageing FUS condensates. Commun Chem 8, 284 (2025). under Attribution 4.0 International License.
Once deposited using this microfluidic approach, the researchers used AFM to obtain detailed topographical and mechanical profiles of individual FUS condensates. Complementary FTIR spectroscopy provided information on secondary structure changes and chemical interactions as the condensates aged. They also performed hydrogen–deuterium exchange experiments to probe how the internal hydrogen bonding networks evolved, offering a molecular view of the transition from liquid-like to solid-like behavior. This combination of microfluidics and nanoscale characterization allowed the team to monitor condensate aging in both time and space, something not possible with traditional bulk techniques.
Their results revealed two distinct and concurrent transformations occurring within FUS condensates. The first was a disorder-to-order transition at the condensate surface, where β-sheet, rich structures emerged, marking the onset of solid-like behavior. The second was a gelation process in the interior, driven by increasing intermolecular interactions between the intrinsically disordered regions of FUS. These processes together led to the progressive stiffening of the condensates: their Young’s modulus increased from a few kilopascals in the early stages to over one megapascal after 24 hours, indicating a gradual solidification over time. The research team found that these changes were accompanied by clear spectral signatures of β-sheet formation and rearrangements in the chemical environment of tyrosine residues, which play a key role in the intermolecular network stabilizing the condensates.
Further molecular insights came from hydrogen–deuterium exchange measurements, which showed a decreasing exchange rate as condensates aged. This indicated that the protein backbones became progressively less flexible, consistent with the formation of denser hydrogen-bond networks. Together, these observations painted a detailed picture of how the initially dynamic and fluid-like FUS condensates transition into more rigid, solid-like states, providing direct evidence of nanoscale heterogeneity and spatially localized phase changes.
In conclusion, this study demonstrates a powerful method to visualize and quantify the molecular aging of protein condensates with unprecedented detail. By combining microfluidic deposition with AFM and FTIR, the researchers created a platform capable of preserving and analyzing delicate condensates in their near-native state. Their findings not only deepen our understanding of the physical processes underlying condensate maturation but also shed light on how changes in molecular organization may contribute to disease-associated protein aggregation. Beyond FUS, this approach offers a valuable framework for exploring phase transitions in other biomolecular systems that govern both healthy and pathological cell function.
“This experimental procedure allowed us to build on the valuable insight provided from structural studies on particular domains, such as the low complexity domain, giving us an understanding of how the overall properties of the full protein sequence may alter the ageing behaviour of FUS condensates31. We also anticipate these methods may be particularly useful for proteins whose phase behaviour is broadly affected by fluorescent tagging, as it does not rely on optical detection.”, the authors concluded.
Figures are reproduced from Miller, A., Toprakcioglu, Z., Qamar, S. et al. Nanoscale profiling of evolving intermolecular interactions in ageing FUS condensates. Commun Chem 8, 284 (2025). https://doi.org/10.1038/s42004-025-01659-z under Creative Commons Attribution 4.0 International License.
Read the original article: Nanoscale profiling of evolving intermolecular interactions in ageing FUS condensates
For more insights into the world of microfluidics and its burgeoning applications in biomedical research, stay tuned to our blog and explore the limitless possibilities that this technology unfolds. If you need high quality microfluidics chip for your experiments, do not hesitate to contact us.


