03 Nov Mapping the “Behaviorome” of Human Neutrophils in a Tumor-on-a-Chip System
Neutrophils, the most abundant immune cells in human blood, play a puzzling dual role in cancer. While some neutrophils (N1) attack tumor cells, others (N2) actually aid tumor progression by supporting invasion and metastasis. Translating insights from animal models to humans has been challenging due to the complexity of the tumor microenvironment and the lack of human-based systems that can capture these cellular interactions in real time. Understanding how N1 and N2 neutrophils behave differently within human tumor tissues could open new possibilities for immunotherapy targeting these cells.
Researchers developed a microfluidic human cell–based microphysiological system called the Neutrophil–Tumor Interactions-on-a-Chip (NTI-chip). This microfluidic device allowed them to recreate a 3D tumor environment using human pancreatic cancer cells and to track how different neutrophil subtypes behave and interact with tumors over time. By quantifying these interactions using their microfluidic chip, the team created a detailed “behaviorome”, a comprehensive profile of neutrophil behaviors, revealing how anti-tumor and pro-tumor neutrophils differ in their actions.

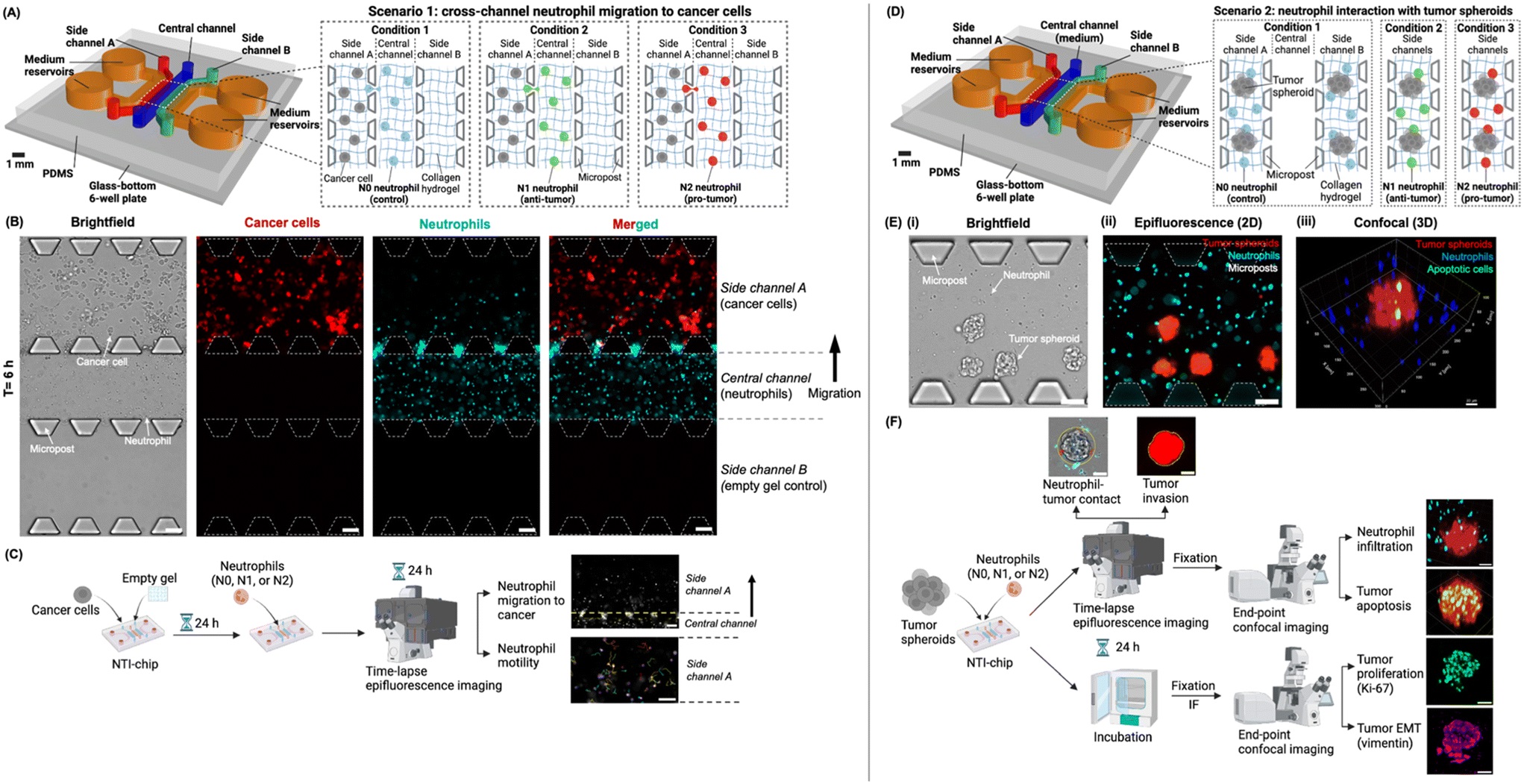
“Experimental design of the “neutrophil–tumor interactions on-a-chip” (NTI-chip) microphysiological system in two independent scenarios. (A) In scenario 1, the NTI-chip models the migration of different human neutrophil subtypes (i.e., N0, N1, or N2 neutrophils) from the central channel into side channel A housing cancer cells, thus achieving spatial compartmentalization. Both neutrophils and cancer cells were embedded in 3D collagen hydrogel to mimic the extracellular matrix of the tumor tissue. Side channel B housed empty hydrogel, acting as a negative control for neutrophil migration. Created with Rhino 7 and https://BioRender.com. (B) Representative 10× brightfield and epifluorescence images showing neutrophils (blue) migrating from the central channel into side channel A housing cancer cells (red) on the NTI-chip at t = 6 h as a representative time point. Scale bar, 100 μm. (C) The workflow of a typical experiment for scenario 1. Neutrophil migration into side channel A and neutrophil motility after migration into side channel A were quantified as readouts. Created with https://BioRender.com. (D) In scenario 2, the NTI-chip models the interaction of N0, N1, or N2 neutrophils with tumor spheroids embedded in 3D collagen hydrogel, which mimics the solid tumor tissue. Side channels A and B are technical replicates. Created with Rhino 7 and https://BioRender.com. (E) Representative 10× brightfield (i) and epifluorescence images (ii) and 3D rendering of 10× confocal images (iii) showing neutrophils (blue) and tumor spheroids (red) in the side channels of the NTI-chip. Apoptotic cells were stained with Caspase-3/7 Green. Scale bar, 100 μm in (ii). X–y–z coordinates are shown around the field of view in (iii). (F) The workflow of a typical experiment for scenario 2 and the behaviors of neutrophils and tumor spheroids that were quantified as readouts. IF, immunofluorescence. EMT, epithelial–mesenchymal transition. Created with https://BioRender.com.” Reproduced from S. Shao, D. Duncko and C. N. Jones, Lab Chip, 2025, Advance Article , DOI: 10.1039/D5LC00526D under Creative Commons Attribution 3.0 Unported Licence.
The microfluidic chip, NTI-chip, was microfabricated using PDMS and featured five microchannels interconnected by microposts, allowing spatially controlled co-culture of neutrophils and cancer cells within a collagen hydrogel that mimics the tumor extracellular matrix. The researchers used HL-60 human promyelocytic cells differentiated into neutrophil-like cells and polarized them into N1-like (anti-tumor) or N2-like (pro-tumor) subtypes using cytokines such as IFN-β, IFN-γ, and TGF-β.
Two experimental setups were implemented:
- Migration Assay: Neutrophils were placed in a central channel, and pancreatic cancer cells in a side channel, enabling visualization of neutrophil migration toward cancer-derived chemokines.
- Tumor Spheroid Interaction: Neutrophils and 3D tumor spheroids were co-embedded in collagen to study contact, infiltration, and tumor progression.
Using live-cell imaging and confocal microscopy, the research team tracked parameters such as migration velocity, contact duration, and infiltration depth, as well as tumor cell responses like invasion, proliferation (Ki-67), and epithelial–mesenchymal transition (vimentin expression) in the microfluidic device.
The study revealed striking behavioral contrasts between neutrophil subtypes. N2-like neutrophils migrated more rapidly toward cancer cells and displayed greater motility once they reached the tumor area. However, N1-like neutrophils formed longer and more sustained contacts with tumor spheroids and penetrated deeper into the 3D tumor mass. Importantly, the presence of N1-like neutrophils reduced tumor invasion, proliferation, and EMT marker expression, while N2-like neutrophils enhanced these cancer-promoting features. Despite their anti-tumor tendencies, N1-like neutrophils exhibited limited tumor-killing capability in 3D cultures, suggesting that the tumor microenvironment may diminish their cytotoxic activity over time.
By integrating microfluidics and human immune-oncology, this study provides a detailed behavioral map of neutrophil subtypes interacting with pancreatic tumors. The proposed microfluidic chip enables real-time, single-cell–level monitoring of immune-tumor dynamics, offering a powerful tool for testing immunotherapeutic strategies. These findings highlight that while N1 neutrophils can suppress tumor progression, more refined modulation or engineering of their functions may be needed to enhance their therapeutic potential. This microfluidic platform holds promise for preclinical testing of immune-targeted therapies and personalized medicine using patient-derived cells.
“The insight into the distinct behavioromes between anti-tumor and pro-tumor neutrophils can guide the development of novel cancer immunotherapies targeting specific neutrophil behaviors. In the future, the microphysiological system can be used to study the extravasation of different neutrophil subtypes into the tumor tissue and test novel therapeutic candidates using patient-derived tumor cells and autologous neutrophils for personalized medicine, thus holding strong potential to facilitate the clinical translation of therapeutics and improve human health”, the authors concluded.
Figures are reproduced from S. Shao, D. Duncko and C. N. Jones, Lab Chip, 2025, Advance Article , DOI: 10.1039/D5LC00526D under Creative Commons Attribution 3.0 Unported Licence.
Read the original article: Behaviorome profiling of anti-tumor and pro-tumor human neutrophil subtypes in a microphysiological system
For more insights into the world of microfluidics and its burgeoning applications in biomedical research, stay tuned to our blog and explore the limitless possibilities that this technology unfolds. If you need high quality microfluidics chip for your experiments, do not hesitate to contact us.


