10 Nov Mimicking Breathing: A Novel Alveoli-on-Chip Model Using Patient-Derived Cells
Recreating the complex mechanical environment of the human lung alveoli in vitro has long been a challenge. Traditional lung-on-chip microfluidic models sometimes fail to fully capture the three-dimensional dynamics of breathing, often relying on overly simplified or non-physiological stretch patterns. Moreover, these systems typically use immortalized or cancer-derived cell lines, which do not accurately represent healthy alveolar epithelial function. A microfluidic device that combines realistic mechanical motion with patient-derived primary cells is essential for advancing lung biology and personalized respiratory research.
Researchers from have developed a novel microfluidic chip, an alveoli-on-chip (AOC) platform that replicates the size, geometry, and cyclic stretching of native alveoli. The system allows three-dimensional deformation to mimic natural respiratory motion and supports the culture of human alveolar epithelial cells derived from patient organoids. This combination enables a physiologically relevant, scalable, and patient-specific microfluidic model of lung mechanics.

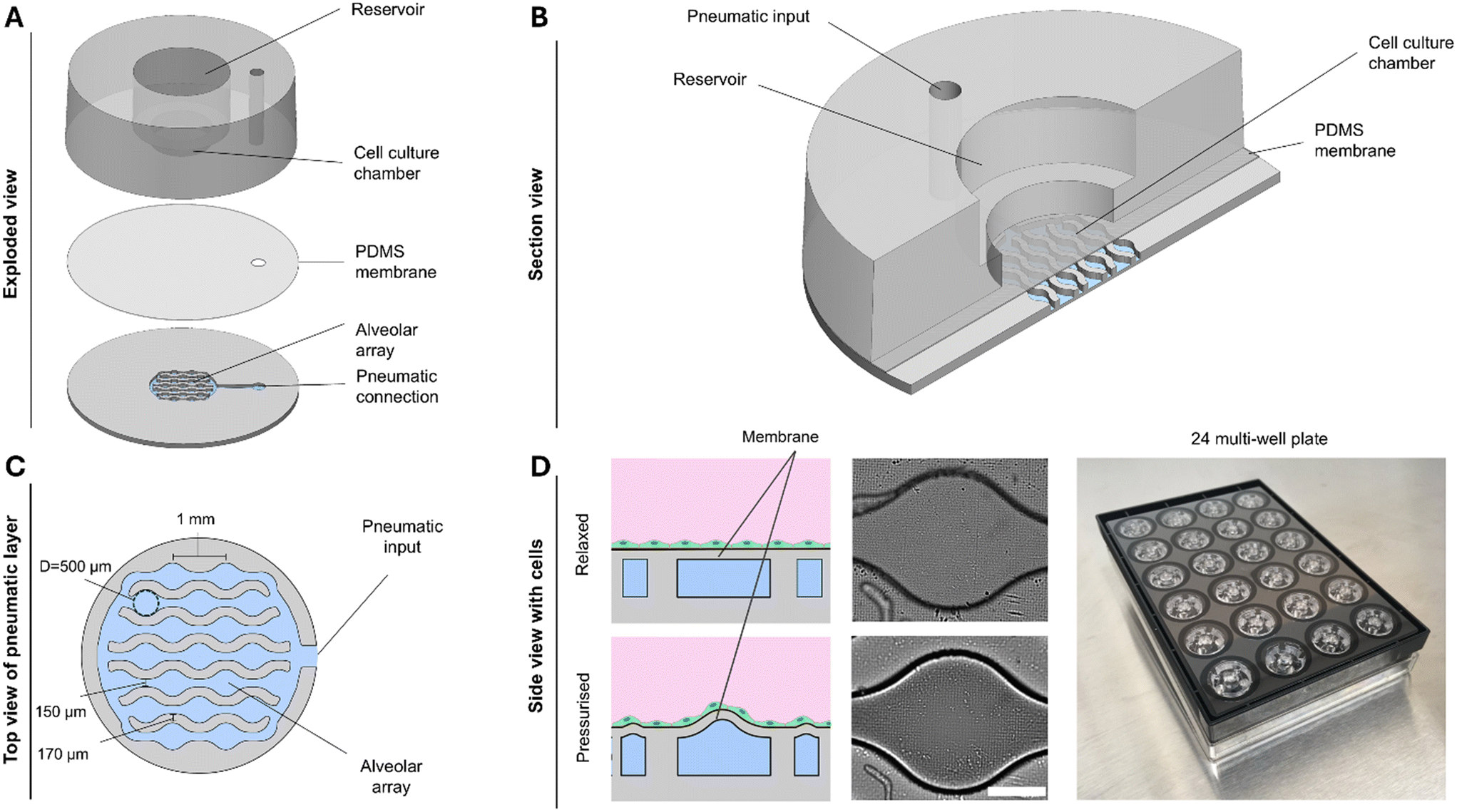
“The novel alveoli-on-chip (AOC). A. Exploded view of the device showing the three main layers: the cell culture chamber with central reservoir, the thin PDMS membrane, and the pneumatically controlled alveolar array with external connection. B. Section view illustrating the apical cell culture chamber, the basal pneumatic input, and the flexible PDMS membrane separating the two compartments. C. Top view of the pneumatic layer showing the interconnected alveolar array with physiologically relevant dimensions (∼500 μm diameter alveoli, 150 μm interconnections). D. Left: Schematic side view showing the membrane with cells in the relaxed state (top) and under positive pressure forming hemispherical protrusions (bottom); center: Microscopy images confirming membrane deformation between relaxed and pressurized states; right: Photograph of the integrated 24-well plate format enabling parallelized experiments and compatibility with standard laboratory workflows.” Reproduced from M. A. Hajari, J. Schulte, D. Principi, D. Schnidrig, S. Schneider, T. Weber, J. Lee, P. Dorn, P. Zamprogno, T. M. Marti and O. T. Guenat, Lab Chip, 2025, Advance Article , DOI: 10.1039/D5LC00473J under Creative Commons Attribution 3.0 Unported Licence.
The microfluidic device consists of three integrated layers, a microfluidic cell culture chamber, a thin flexible PDMS membrane, and a pneumatic chamber that drives cyclic pressure changes. The microfluidic chip’s sinusoidal design produces interconnected circular alveoli approximately 500 μm in diameter, arranged to reproduce natural curvature and strain gradients. When cyclic pressure is applied, the membrane deforms upward, simulating the expansion of alveoli during breathing.
To provide relevant human cells, type II alveolar epithelial (AT2) cells were isolated from patient lung tissues, expanded as organoids, and later seeded onto the AOC. The researchers optimized organoid growth by supplementing the culture medium with endothelial-derived factors, increasing organoid size and yield by approximately 2.5-fold. Once seeded on-chip, these cells formed a confluent epithelial layer and were subjected to cyclic stretch at 0.25 Hz for 24 hours—approximating normal breathing frequency.
The AOC microfluidic platform demonstrated precise and stable mechanical performance, with membrane deflections closely matching computational models. The team verified that the system could reproduce physiologically relevant 10% linear strain with minimal drift over time. RNA sequencing revealed that cyclic stretch significantly altered gene expression in patient-derived alveolar cells, upregulating pathways related to autophagy, mTORC1 signaling, and cell cycle regulation while downregulating genes associated with inflammation, fatty acid metabolism, and epithelial–mesenchymal transition. These findings indicate that mechanical breathing cues influence cellular homeostasis and maturation in ways consistent with native lung physiology.
The microfluidic study revealed striking behavioral contrasts between neutrophil subtypes. N2-like neutrophils migrated more rapidly toward cancer cells and displayed greater motility once they reached the tumor area. However, N1-like neutrophils formed longer and more sustained contacts with tumor spheroids and penetrated deeper into the 3D tumor mass. Importantly, the presence of N1-like neutrophils reduced tumor invasion, proliferation, and EMT marker expression, while N2-like neutrophils enhanced these cancer-promoting features. Despite their anti-tumor tendencies, N1-like neutrophils exhibited limited tumor-killing capability in 3D cultures, suggesting that the tumor microenvironment may diminish their cytotoxic activity over time.
This study presents a robust and scalable alveoli-on-chip microfluidic platform capable of replicating human alveolar breathing dynamics using patient-derived cells. The model provides a physiologically faithful microenvironment for studying mechanobiological responses, disease mechanisms, and drug effects in the lung. By integrating organoid technology with microengineering, the AOC opens new possibilities for personalized respiratory research and preclinical testing.
“Beyond advancing our fundamental understanding of alveolar mechanobiology, this integrated platform of patient derived alveolospheres and organ-on-chip technology offers promising potential for personalized medicine applications. The ability to culture patient-specific alveolar epithelial cells under physiologically relevant mechanical conditions could enable personalized drug screening, disease modeling, and therapeutic optimization tailored to individual patient responses.”, the authors concluded.
Figures are reproduced from M. A. Hajari, J. Schulte, D. Principi, D. Schnidrig, S. Schneider, T. Weber, J. Lee, P. Dorn, P. Zamprogno, T. M. Marti and O. T. Guenat, Lab Chip, 2025, Advance Article , DOI: 10.1039/D5LC00473J Creative Commons Attribution 3.0 Unported Licence.
Read the original article: A novel alveoli-on-chip platform for modeling cyclic stretch in patient-derived alveolar epithelial cells cultured from organoids
For more insights into the world of microfluidics and its burgeoning applications in biomedical research, stay tuned to our blog and explore the limitless possibilities that this technology unfolds. If you need high quality microfluidics chip for your experiments, do not hesitate to contact us.


