15 Jan Rapid culture-free pathogen diagnosis using microfluidics and Raman spectroscopy
Timely identification of infectious pathogens remains a major bottleneck in clinical care, particularly in conditions such as sepsis or complicated urinary tract infections where every hour of delay can worsen outcomes. Conventional diagnostics rely on culture-based workflows that often take days, struggle with low pathogen loads, and encourage empirical use of broad-spectrum antibiotics. This delay contributes directly to inappropriate therapy and the growing burden of antimicrobial resistance.
In this study, a culture-free microfluidic diagnostic pipeline is developed that compresses the entire workflow from raw clinical sample to pathogen identification into less than 20 minutes. The microfluidic device combines automated microfluidic enrichment, single-cell Raman micro-spectroscopy, and deep learning-based classification to enable rapid, untargeted detection of bacterial and fungal pathogens directly from patient samples.
“Current diagnosis is hindered by prolonged culturing and difficulties detecting low pathogen loads. Here, we present a culture-free diagnostic platform that integrates microfluidics, Raman micro-spectroscopy, and deep learning to deliver “sample-to-report” testing within 20 min”, the authors explained.
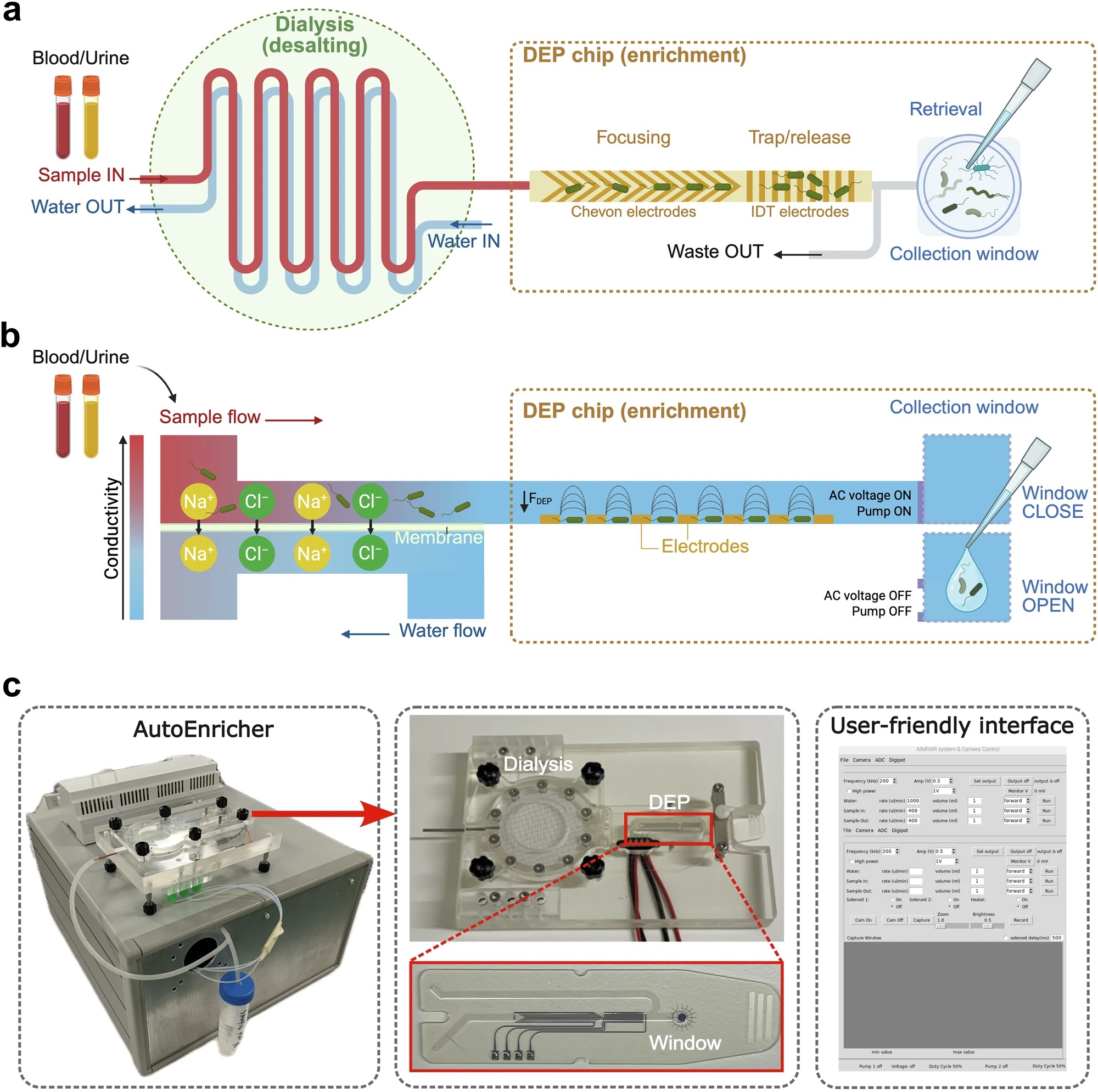
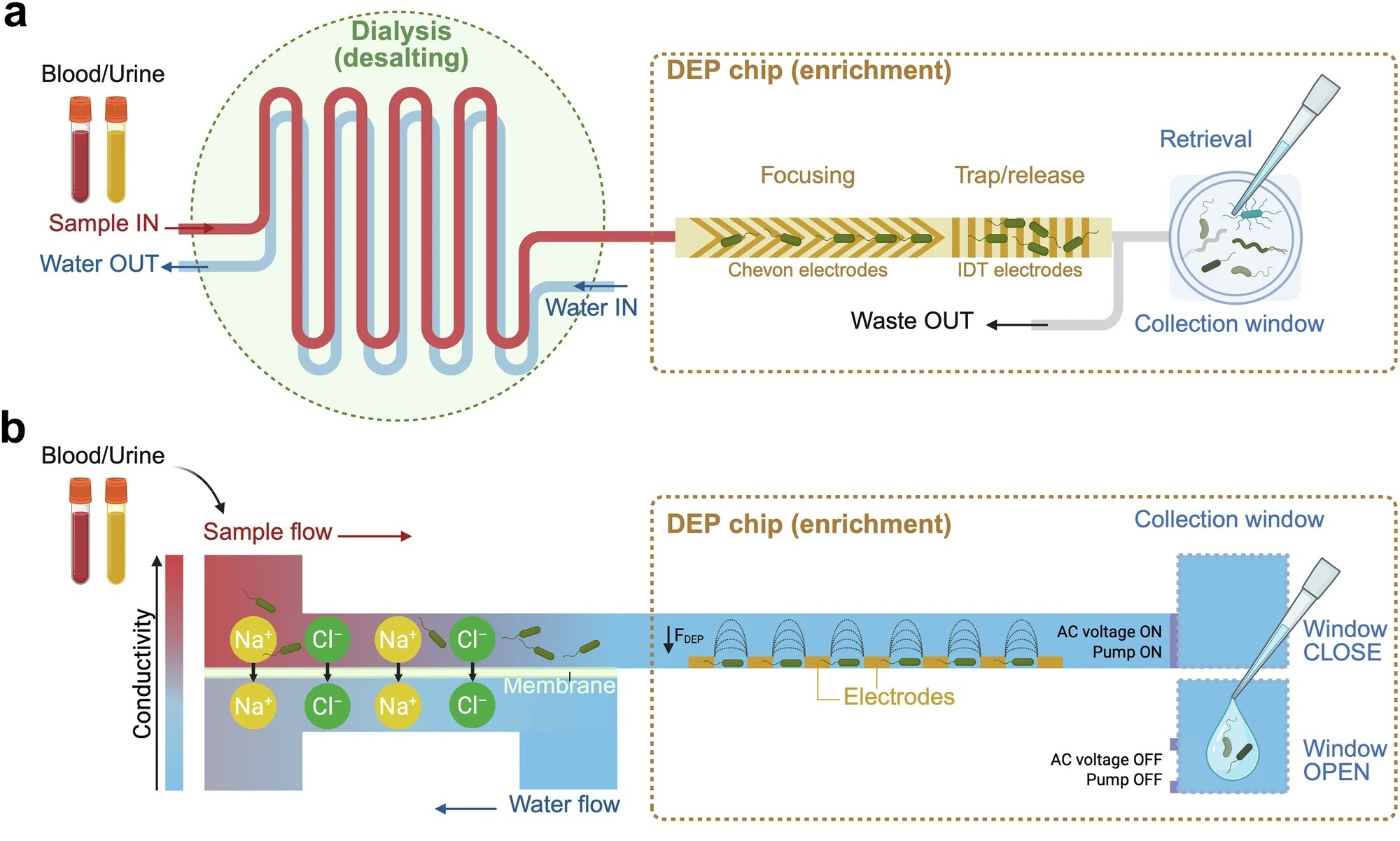
“a Top view and (b) Side view of the system. The dialysis unit consists of two layers of microfluidic channels separated by a porous membrane. As the sample flow passes through the upper channel, electrolytes diffuse through the membrane and are removed by the counterflow of water below. This desalted sample flow then enters the DEP chip, where pathogens are focused in the centre of the channel and trapped on the IDT arrays due to positive dielectrophoresis (pDEP) forces. When the AC voltage is turned off and the pump is stopped, the captured cells are released into the collection window for in situ or off-chip analysis. Created in BioRender. Xu, J. (https://BioRender.com/038g1ru). c Photo of the enrichment system (AutoEnricher–a compact benchtop instrument that houses all components). The dialysis unit and DEP chip are disposable components housed in a customised plastic base. A user-friendly control software enables automated processing after sample loading.” Reproduced from Li, Y., Xu, J., Yi, X. et al. Rapid culture-free diagnosis of clinical pathogens via integrated microfluidic-Raman micro-spectroscopy. Nat Commun 17, 283 (2026). under a Creative Commons Attribution 4.0 International License.
At the core of their solution is an automated microfluidic chip that isolates pathogens from complex clinical matrices without affinity labels or prior knowledge of species. The microfluidic platform integrates membrane dialysis with positive dielectrophoresis to first reduce sample conductivity and then selectively trap microbial cells. This approach allows efficient processing of millilitre-scale samples and achieves capture efficiencies above 90 percent across Gram-negative bacteria, Gram-positive bacteria, and clinically relevant fungi. Importantly, the system maintains performance at extremely low pathogen concentrations, with demonstrated capture down to fewer than 2 colony-forming units per millilitre.
Once enriched, the captured cells are released into a small droplet and transferred directly onto a Raman slide for analysis. Single-cell Raman spectroscopy provides a biochemical fingerprint of each cell based on its intrinsic molecular composition, removing the need for stains or genetic amplification. The authors built an extensive Raman database consisting of more than 100,000 single-cell spectra collected from 342 clinical isolates representing 36 bacterial and fungal species commonly encountered in hospitals. These spectral fingerprints were used to train a one-dimensional ResNet deep learning model capable of classifying pathogens at the species level.
In laboratory validation, the trained model achieved an average species-level accuracy of 95.1 percent across the full isolate database. Reliable identification required only a small number of spectra per sample, enabling Raman acquisition to be completed in a few minutes and fitting seamlessly within the overall rapid workflow. The model was further adapted using a fine-tuning dataset derived from real clinical urine samples to improve performance under the variability encountered in patient specimens.
The clinical relevance of the microfluidic platform was demonstrated in a prospective study involving 305 patient samples, primarily urine but also including bile, cerebrospinal fluid, and drainage fluids. Using optical inspection after enrichment to determine infection status, the system achieved an overall diagnostic accuracy of 95.4 percent, with a sensitivity of 98.5 percent when compared to standard culture-based methods. Pathogen identification by Raman spectroscopy showed strong agreement with MALDI-TOF results, including successful detection of mixed bacterial and fungal infections that are often missed or underreported by culture.
In conclusion, this work shows how automated microfluidic enrichment combined with single-cell Raman spectroscopy and deep learning can enable rapid, culture-free pathogen diagnosis at clinically relevant sensitivities. By reducing time to identification from days to minutes and preserving broad-spectrum detection, the approach addresses a key unmet need in infectious disease diagnostics and antibiotic stewardship. With further validation across diverse sample types and integration with antimicrobial susceptibility testing, this microfluidic platform could play an important role in future clinical workflows
“In conclusion, our approach is simple to operate, requiring no culture or destructive processing, and offers a rapid “sample-to-report” workflow within 20 min, meeting the urgent diagnostic need for rapid identification in ICUs and in the primary care where timely detection of bacterial infections is critical. It represents a significant advancement in clinical microbiology.”, the authors concluded.
Figures are reproduced from Li, Y., Xu, J., Yi, X. et al. Rapid culture-free diagnosis of clinical pathogens via integrated microfluidic-Raman micro-spectroscopy. Nat Commun 17, 283 (2026). https://doi.org/10.1038/s41467-025-66996-y under a Creative Commons Attribution 4.0 International License.
Read the original article: Rapid culture-free diagnosis of clinical pathogens via integrated microfluidic-Raman micro-spectroscopy
For more insights into the world of microfluidics and its burgeoning applications in biomedical research, stay tuned to our blog and explore the limitless possibilities that this technology unfolds. If you need high quality microfluidics chip for your experiments, do not hesitate to contact us.


