17 Jun A Reconfigurable Microfluidic Platform for Sample-Efficient Antibody Fc Biomarker Discovery
Precise diagnosis of infectious diseases is often hindered by a lack of accessible biomarkers that can distinguish between active and past infections. While antibody levels are commonly measured, they typically fail to provide this distinction, especially in endemic contexts where antibodies can persist long after the infection has cleared. Among newer candidates, the fragment crystallizable (Fc) region of antibodies, particularly its glycosylation state and subclass, is emerging as a more informative marker. However, standard approaches for Fc biomarker discovery are limited by high sample requirements, specialized protocols, and cost.
To address this, researchers have employed microfluidics technology. They developed a cleanroom-free, reconfigurable microfluidic chip, termed the μMAP (Microfluidic Multiplexed Antibody-omic Profiling) platform, that enables simultaneous profiling of antibody Fab and Fc properties from clinical serum samples using just 15 μL of input. The microfluidic platform integrates an antigen microarray with removable PDMS-based channels, allowing for flexible sample and probe routing. The microfluidic device performs 1400 assays in a single run, capturing 100 antibody features per sample, including antigen specificity, isotype, subclass, glycosylation, and Fc receptor affinity.
“Here we present a simple, flexible and reconfigurable microfluidic device, made using rapid prototyping techniques, that can perform highly multiplexed and high-throughput biomarker discovery targeting both antibody fragment antigen-binding (Fab) and Fc features including antigen specificity, antibody isotypes, subclasses, N-glycosylation and Fc receptor binding.“, the authors explained.
The μMAP chip was fabricated using rapid prototyping techniques such as laser-cutting of PDMS sheets and printed antigen microarrays. Channels were manually aligned without permanent bonding, and the assay proceeded through two reconfigurable incubation steps, first for sample exposure, then for fluorescent probe binding. Signal detection was achieved using a microarray scanner or fluorescence microscopy, depending on equipment availability.

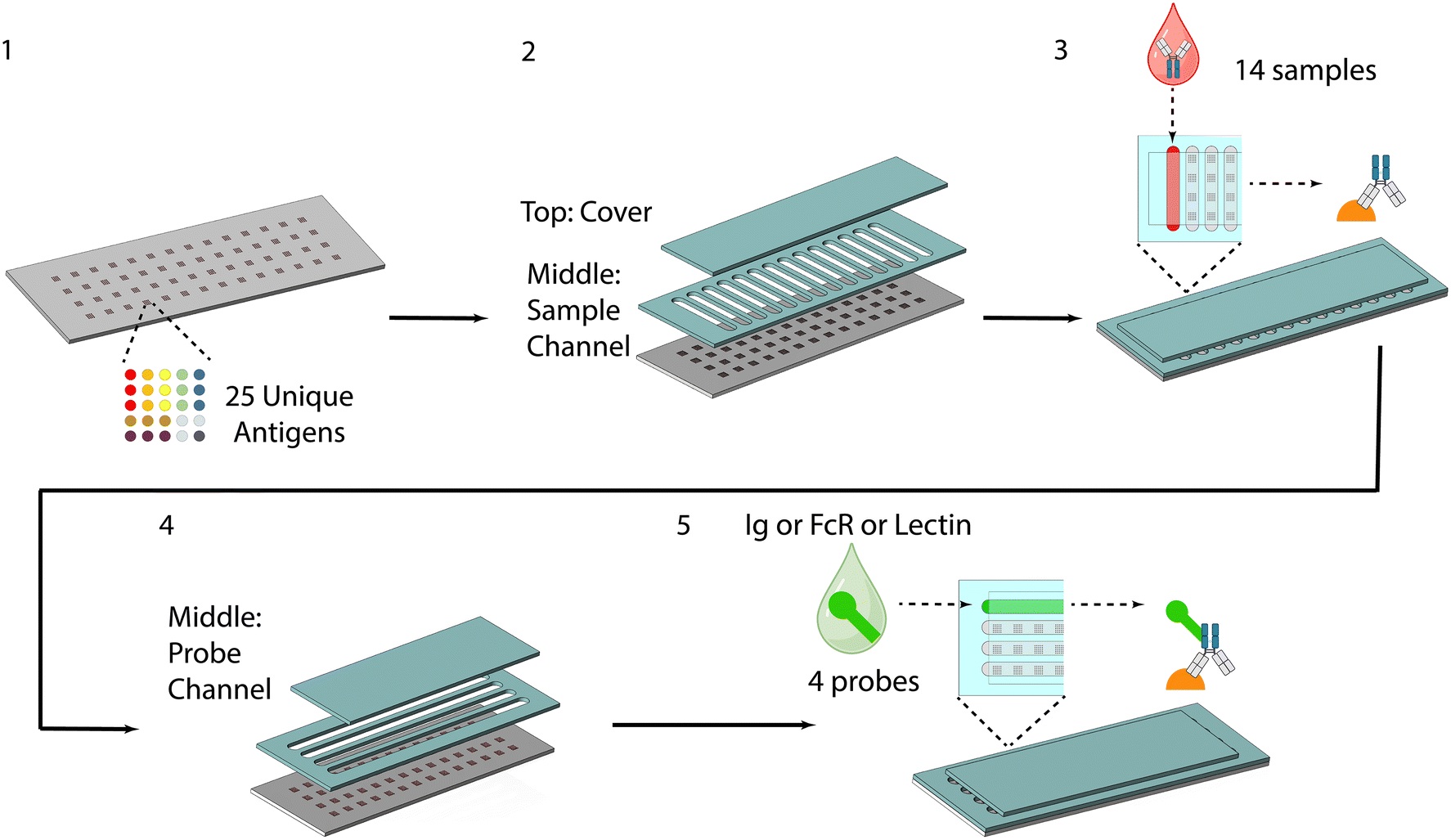
“Schematic of the workflow of the device, including: 1. microarray printing, 2. sample channel attachment, 3. sample addition, incubation and removal, 4. probe channel attachment, 5. probe addition, incubation and removal.” Reproduced from H. Zhang, D. Bhakta, A. Saha, S. P. Peddireddy, S. Bao, L. Li, S. Handali, W. E. Secor, L. A. O. Fraga, J. K. Fairley and A. Sarkar, Lab Chip, 2025, 25, 2828 DOI: 10.1039/D5LC00042D with permission from Royal Society of Chemistry.
In benchmark testing, the μMAP platform demonstrated specific, reproducible binding across antigens and probes, with sensitivity on par with ELISA and dynamic ranges spanning over three orders of magnitude. Importantly, it accurately measured glycosylation patterns using lectin-binding assays, correlating strongly with LC-MS results, and successfully profiled Fc receptor binding.
For a real-world application, the device was used to analyze serum samples from individuals with current or past schistosomiasis infections. While traditional antibody levels could not distinguish between active and former infections, SEA-specific IgG4 levels measured on the μMAP platform clearly differentiated these states. The results were consistent with parallel Luminex bead-based assays, validating the platform’s reliability.
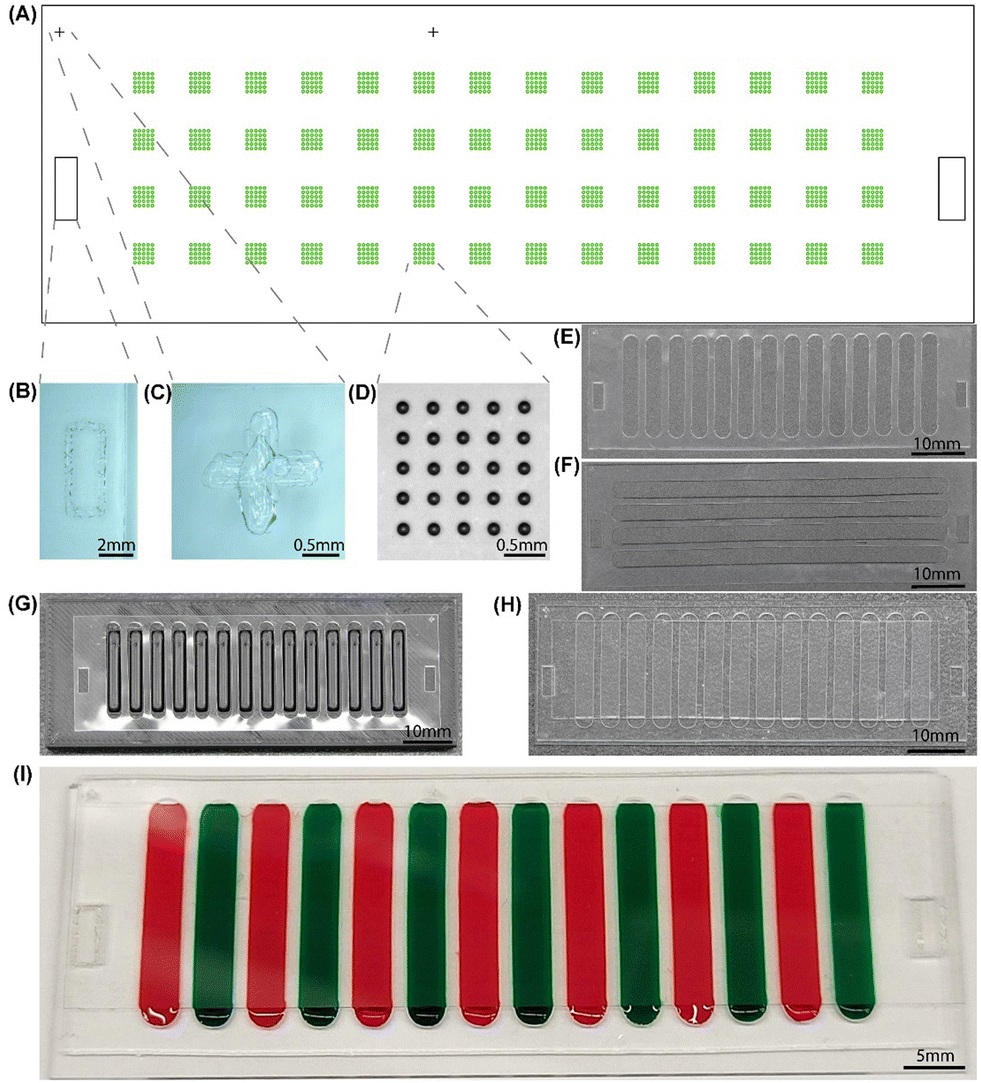
“Physical design of the device. (A) 2D layout of alignment marks and microarrays on the glass slide. (B) Optical image of an alignment mark used by PDMS attachment. (C) Optical image of an alignment mark used by microarray printing. (D) Optical image of a 5 × 5 array after printing. (E) PDMS middle layer for sample. (F) PDMS middle layer for probes. (G) Sample middle layer rectified on 3D printed alignment block. (H) Optical image of the device after full assembly. The sample version was used as the middle layer. (I) Demonstration of the assembled device with colored dye filling the channels as samples.” Reproduced from H. Zhang, D. Bhakta, A. Saha, S. P. Peddireddy, S. Bao, L. Li, S. Handali, W. E. Secor, L. A. O. Fraga, J. K. Fairley and A. Sarkar, Lab Chip, 2025, 25, 2828 DOI: 10.1039/D5LC00042D with permission from Royal Society of Chemistry.
In conclusion, the μMAP device offers a low-cost, low-volume, and highly multiplexed method for antibody Fc biomarker discovery. Its ease of use and minimal sample demands make it especially promising for use in clinical labs and field settings, particularly in regions with limited resources. The platform holds potential for broad application across infectious diseases, autoimmunity, and vaccine response monitoring.
“Future work includes performing biomarker discoveries for other endemic diseases in the field or in other laboratories, increasing the number of samples that can be tested per device by modifying channel width or substrate size, incorporating pre-printed probes and further improving usability by incorporating feedback from user studies.“, the authors concluded
Figures are reproduced from H. Zhang, D. Bhakta, A. Saha, S. P. Peddireddy, S. Bao, L. Li, S. Handali, W. E. Secor, L. A. O. Fraga, J. K. Fairley and A. Sarkar, Lab Chip, 2025, 25, 2828 DOI: 10.1039/D5LC00042D with permission from Royal Society of Chemistry.
Read the original article: Sample-sparing multiplexed antibody Fc biomarker discovery using a reconfigurable integrated microfluidic platform
For more insights into the world of microfluidics and its burgeoning applications in biomedical research, stay tuned to our blog and explore the limitless possibilities that this technology unfolds. If you need high quality microfluidics chip for your experiments, do not hesitate to contact us.


