27 May Compartmentalized perfusion enables precise control of microenvironments in cardiac microfluidics
In complex tissue environments, cells constantly interact with dynamic chemical signals, many of which are transported by blood flow. Reproducing these in vitro without oversimplifying the microenvironment remains a core challenge in organ-on-chip systems. The authors of this study sought to address this by engineering a microfluidic platform that allows spatially confined, time-controlled delivery of soluble factors to cardiomyocytes derived from human induced pluripotent stem cells (hiPSC-CMs).
To overcome the limitations of conventional perfusion systems, such as shear stress that can disturb excitable cells like cardiomyocytes, the authors created an open-top, compartmentalized microfluidic device. This design includes a perfusion channel separated from the main cell culture area by triangular pillars and microtunnels. These microfluidic features maintain chemical separation while allowing cell interaction and enable flow to be directed through a specific microfluidic compartment without affecting the others. By using gravity-driven and pump-controlled flow, the authors mimicked the low-shear, slow interstitial flow typical of soft tissues.
“In this paper, we propose an open-top organ-on-chip structure with compartment-specific perfusion to introduce stimulating molecules to cells with only minimal extra unspecific stimulus. Using numerical simulations, we show that shear stress sensed by the cells within the structure is low. We further validated the flow profile experimentally. “, the authors explained.
Standard organ-on-chip models often suffer from two key limitations. First, perfusion is typically applied uniformly across a culture chamber, leading to global chemical exposure and uniform shear stress. Second, microfluidic designs that achieve spatial separation of cell types tend to lack flexible flow control or are too complex to scale up. For hiPSC-derived cardiomyocytes (hiPSC-CMs), which are sensitive to both flow-induced shear and sudden medium changes, these constraints limit physiological relevance and reproducibility in drug screening.
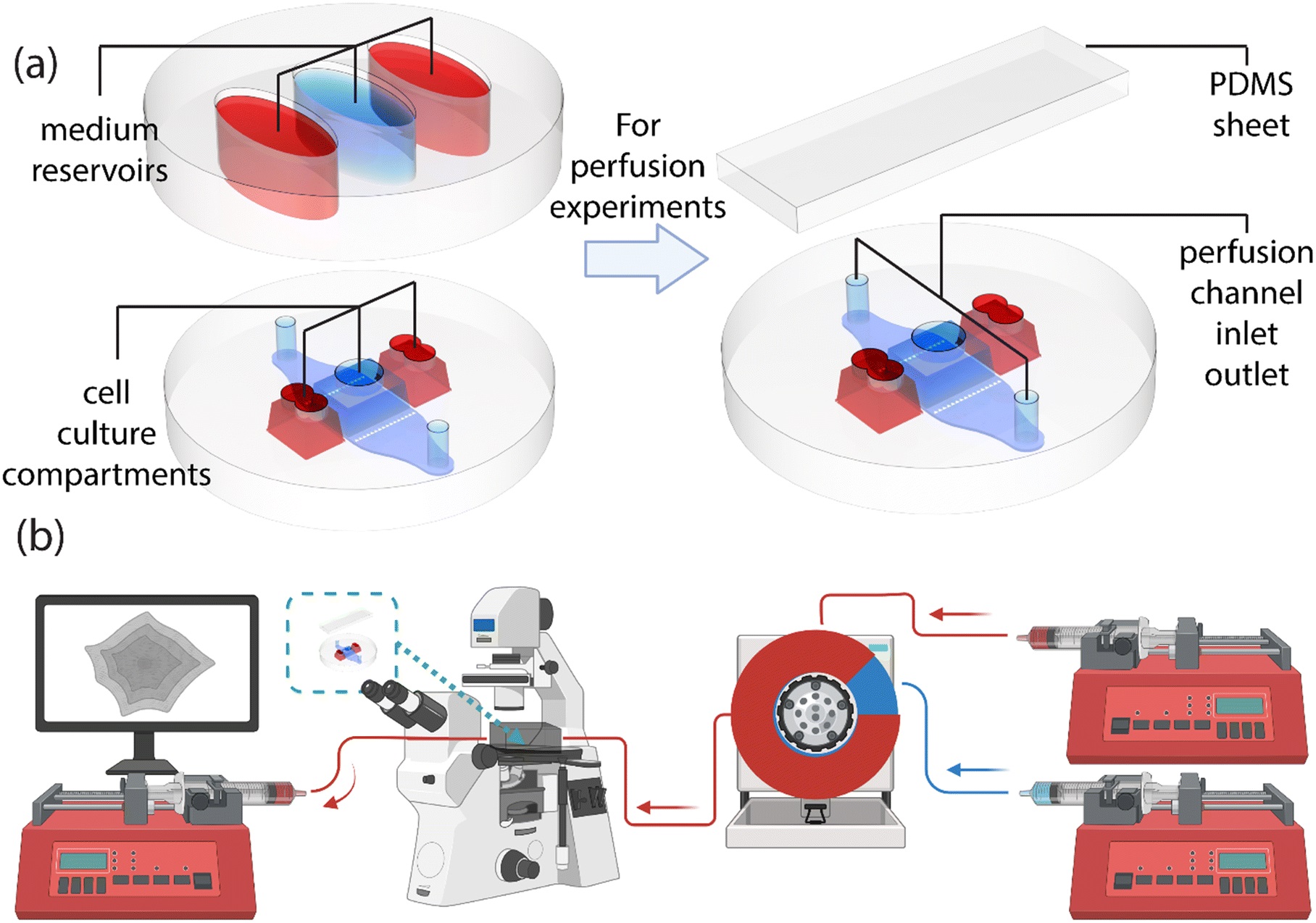
“The chip structure and the system setup for observing the beating of cardiomyocytes under continuous perfusion. (a) The base of the chip (at the left and right lower corners) includes the cell culture compartments and the perfusion channel, which can be accessed through the openings on top. These openings enable cell seeding and provide access to medium reservoirs (left top corner) during stationary experiments. The medium reservoirs increase the volume of the cell culture medium, further supporting cell culture in these compartments. Once the chip is used for perfusion experiments, the medium compartment is replaced with a sheet of PDMS (top right corner) to seal the openings and to form a closed channel. (b) The inlet side of the perfusion setup consists of two syringe pumps; one filled with culture medium and the other with culture medium including 1 μM adrenaline, both connected to a recirculation valve. The valve injects adrenaline to the inlet stream of the chip. The chip is placed into an incubator box, where temperature and 19% oxygen and 5% carbon dioxide levels are maintained constant throughout the experiments. A third syringe pump withdraws medium from the chip aiding the medium supply through the chip. Beating of the human-induced pluripotent stem cell-derived cardiomyocytes is recorded in different time points with a camera attached to an optical microscope.” Reproduced from K. Tornberg, W. Grötsch, N. Ritari, S. Haikka, L. Sukki, K. Aalto-Setälä, M. Pekkanen-Mattila and P. Kallio, Lab Chip, 2025, Advance Article, DOI: 10.1039/D5LC00072F under a Creative Commons Attribution 3.0 Unported Licence.
The authors microfabricated a PDMS-based, open-top microfluidic chip composed of three culture compartments arranged horizontally and connected via arrays of narrow microfluidic microtunnels. This layout supports both compartmentalized microfluidic culture and direct cell–cell interaction. Perfusion is localized to the center microfluidic compartment using a dedicated perfusion channel that is isolated from the culture area by triangular micropillars. These features ensure that perfusion flow affects only the targeted compartment, while maintaining chemical diffusion and mechanical isolation from the adjacent chambers.
Human iPSC-derived cardiomyocytes were seeded into the central compartment of the microfluidic device, and the platform was maintained in a controlled environment mimicking physiological temperature, CO₂, and O₂ levels. A recirculation loop, comprising two syringe pumps and a valve system, allowed researchers to switch between plain culture medium and medium containing 1 µM adrenaline. Beating patterns of the cardiomyocytes were monitored in real-time using live imaging and motion tracking software. The authors recorded cellular contractions before, during, and after adrenaline stimulation, capturing the immediate effects of chemical changes delivered via flow.
Under continuous flow of control medium (without adrenaline), the beating rate of hiPSC-CMs remained stable, confirming that the applied shear stress was minimal and well tolerated. Upon introduction of adrenaline through perfusion, the beating rate increased significantly, with cells exhibiting a dose and time-dependent response similar to what is observed in vivo during adrenergic stimulation. After switching back to control medium, the beating rate gradually returned to baseline, demonstrating reversible and temporally confined stimulation. Importantly, adjacent compartments that were not exposed to perfused adrenaline did not show changes in beating behavior, underscoring the spatial confinement of stimulation.
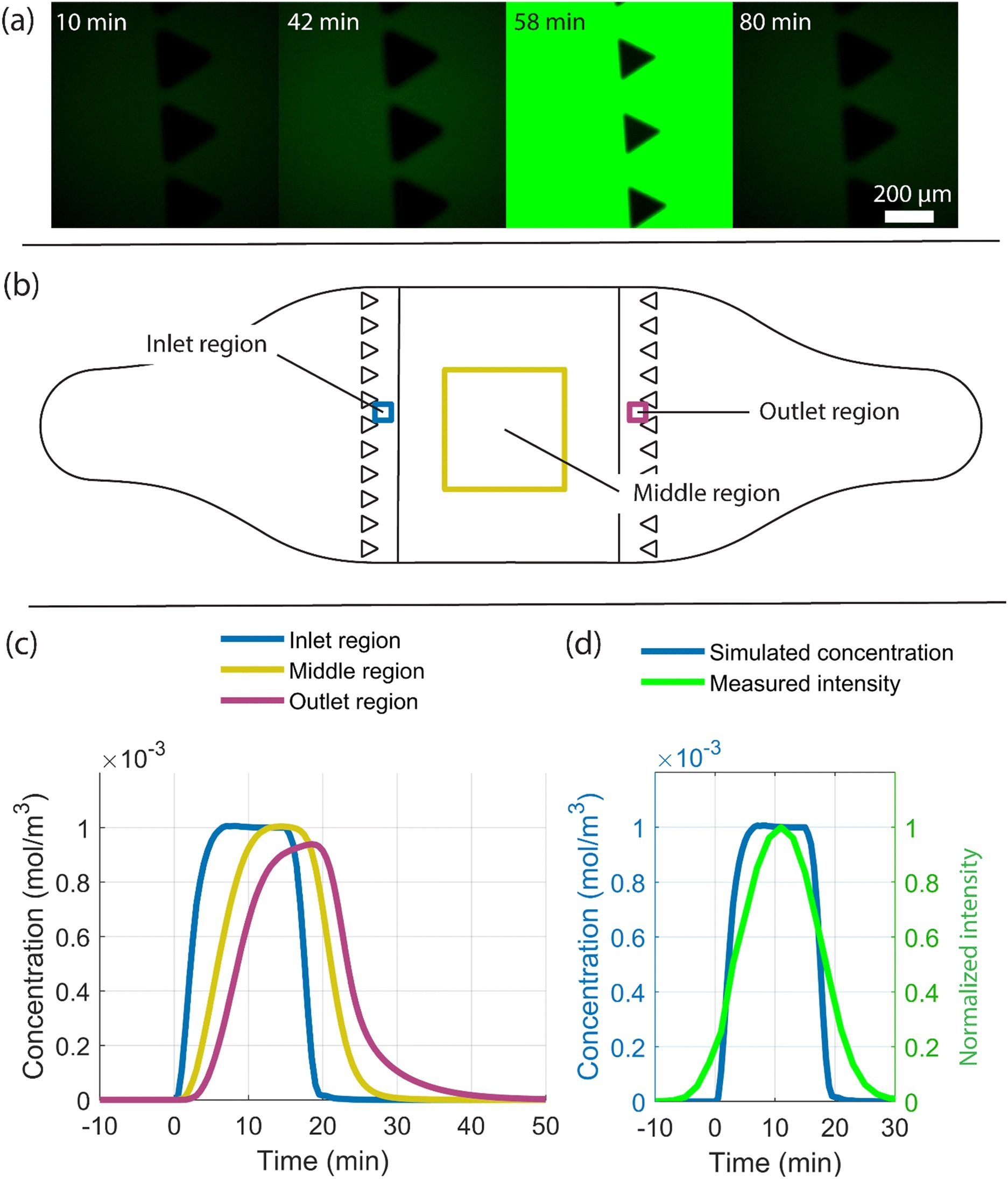
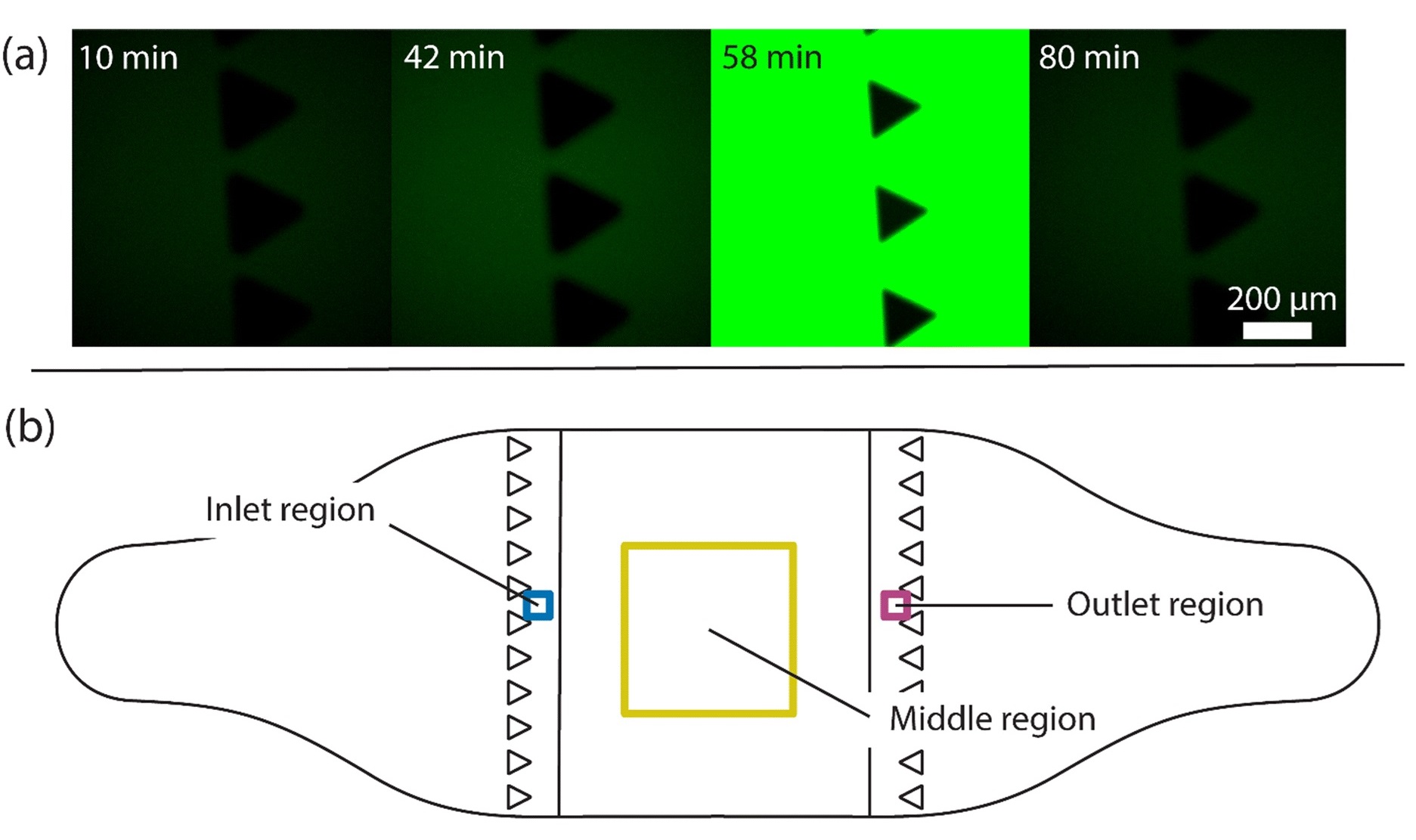
“Study of the arrival of molecules provided by the flow to the chip and the cells adhering to the cell culture area. (a) Fluorescent images at four time points where fluorescein reaches the chip inlet pillars after a 15-minute injection followed by flushing. Measurements were conducted to determine the time delay between switching the valve and arrival of a substance at the cell culture compartment. At 42 minutes after switching the valve, the first fluorescein molecules arrive at the pillar area, indicated by a higher intensity, higher than that in the reference image (the 10-minute time point). The highest fluorescein intensity is seen at 58 minutes, and by 80 minutes, all fluorescein molecules are flushed out. A 15-minute injection of 1 μM adrenaline followed by a flush was simulated and the average adrenaline concentration over time in three regions as shown in (b) were studied. The regions include the inlet (blue) and outlet (magenta) regions with a 150 μm × 150 μm area and the middle region (yellow) with a 2 mm × 2 mm area. (c) Plot of the simulated arrival of adrenaline molecules in the inlet, middle and outlet regions, and (d) a comparison of the simulated arrival of adrenaline molecules (blue) at the inlet region with the measured arrival of fluorescein molecules shown as normalized intensity (green) over time.” Reproduced from K. Tornberg, W. Grötsch, N. Ritari, S. Haikka, L. Sukki, K. Aalto-Setälä, M. Pekkanen-Mattila and P. Kallio, Lab Chip, 2025, Advance Article, DOI: 10.1039/D5LC00072F under a Creative Commons Attribution 3.0 Unported Licence.
This study demonstrates that compartmentalized perfusion can effectively deliver chemical stimuli to defined regions within a microfluidic chip, providing a powerful approach for modeling localized drug responses in sensitive cell types such as cardiomyocytes. By preserving the mechanical integrity of the culture environment and offering fine temporal control over drug exposure, this platform bridges an important gap between in vitro systems and the complex, dynamic signaling environments found in human tissues.
“The current version of the structure still faces challenges with interfacing, as Taylor dispersion was seen to disturb the direct comparison of the numerical model with the real-life measurements. Additionally, while the use of syringe pumps allows for the establishment of steady flow conditions within the chip and, together with the valve system, offers precise temporal control of the cellular microenvironment, these pieces of equipment may pose limitations for certain studies. The chip, combined with human-based cells, can be used for drug studies to gain a better understanding of the dynamics, effects and responses of drugs in target tissue within the human body.“, the authors concluded
Figures are reproduced from K. Tornberg, W. Grötsch, N. Ritari, S. Haikka, L. Sukki, K. Aalto-Setälä, M. Pekkanen-Mattila and P. Kallio, Lab Chip, 2025, Advance Article , DOI: 10.1039/D5LC00072F under a Creative Commons Attribution 3.0 Unported Licence.
Read the original article: Compartmentalized perfusion for temporal control of the chemical microenvironment of iPSC-derived cardiac cells
For more insights into the world of microfluidics and its burgeoning applications in biomedical research, stay tuned to our blog and explore the limitless possibilities that this technology unfolds. If you need high quality microfluidics chip for your experiments, do not hesitate to contact us.


