26 Jan Droplet microfluidics for deep mutational scanning
Caspases, a family of protease enzymes, play a crucial role in numerous biological processes, making them intriguing subjects of study for researchers. Understanding the structure, function, and regulation of caspases could pave the way for innovative therapeutic approaches. In a groundbreaking research study, scientists embarked on a journey to unravel the functional differences between closely related caspase variants. Using high-throughput screening and microfluidic techniques, they delved into the intricate world of caspases, shedding light on their activities and potential applications.
“Our approach leverages high-throughput microfluidic screening to analyze hundreds of thousands of caspase variants in tightly controlled in vitro reactions. The resulting data provides a large-scale and unbiased view of the impact of amino acid substitutions on the proteolytic activity of CASP3 and CASP7. We use this data to pinpoint key functional differences between CASP3 and CASP7, including a secondary internal cleavage site, CASP7 Q196 that is not present in CASP3. “, the authors explained

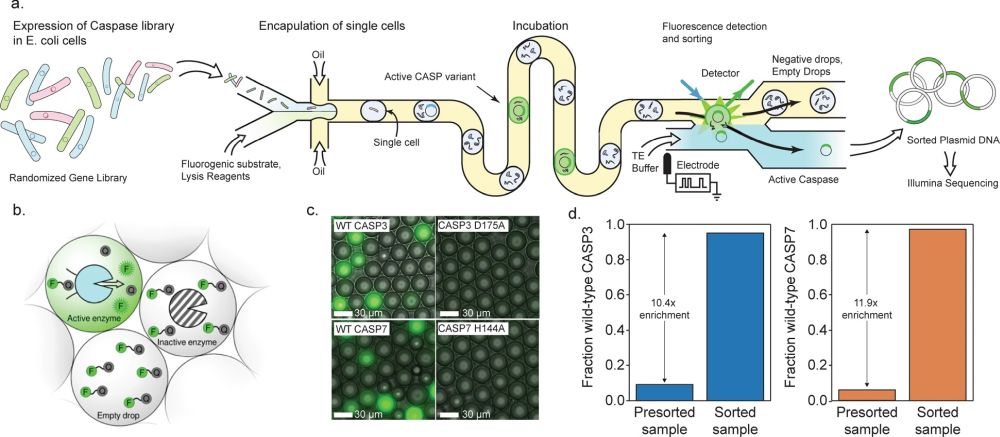
“a A schematic of our microfluidic screening system. A dilute suspension of E. coli expressing caspase variants are injected into a microfluidic device and individual cells are encapsulated into microdroplets containing lysis reagents and a fluorogenic caspase substrate. The cells are lysed, the enzyme reaction is incubated on-chip, and the fluorescence of each droplet is analyzed using a laser. The fluorescent droplets are then sorted by electrocoalescence with an aqueous stream that collects the sorted plasmids for downstream analysis. b Droplets containing active caspase variants will fluoresce, whereas empty droplets and droplets containing inactive caspases will not. c Microscopy images of droplets containing active WT CASP3 and WT CASP7 display strong green fluorescence, while droplets containing the inactive CASP3 D175A and CASP7 H144A variants remain dark. d Results of a mock screen demonstrate over tenfold enrichment of active CASP3 and CASP7.” Reproduced under Creative Commons Attribution 4.0 International License from Roychowdury, H., Romero, P.A. Microfluidic deep mutational scanning of the human executioner caspases reveals differences in structure and regulation. Cell Death Discov. 8, 7 (2022). https://doi.org/10.1038/s41420-021-00799-0
The research team leveraged a high-throughput droplet microfluidic screening platform, which allowed them to analyze over 300,000 variants per hour in a controlled in vitro environment. This platform offered several advantages, including precise control over reaction timescales, enabling the differentiation of caspase variants with altered activity. In comparison to cell-based assays, the microfluidic device provided faster reaction timescales, distinguishing finer functional differences among the variants.
The researchers mapped the effects of a vast number of amino acid substitutions in two caspase variants: CASP3 and CASP7. Approximately one-third of all possible single amino acid substitutions were analyzed, providing valuable insights into mutational tolerance and constraints within the enzymes. The findings aligned with previous research, confirming the mutational intolerance of catalytic cysteine and histidine residues and highlighting known allosteric and processing sites. Moreover, the study revealed mutational constraints that followed established understanding of protein stability, such as the impact of disrupting salt bridges or mutations in the hydrophobic core.
Comparing the mutational profiles of CASP3 and CASP7, the researchers identified sites with statistically significant differences in mutational tolerance. While the majority of sites displayed similar mutational tolerance, a small subset stood out. Notably, CASP7 exhibited a structurally crucial salt-bridge network absent in CASP3. This finding opens up possibilities for designing selective caspase inhibitors by targeting this network, which could have implications in drug design and the development of novel therapeutics.
The study provided valuable insights into the functional differences between closely related caspase variants, highlighting potential sites for future drug design and therapeutic interventions. The research also emphasized the limitations of the study, including the use of E. coli expression systems lacking native regulatory partners and the assessment of caspase activity on a single fluorogenic substrate. Further biochemical characterization is necessary to obtain a comprehensive understanding of the molecular mechanisms underlying caspase activity.
The study’s results pave the way for future research directions, such as investigating the role of residue Q196 in CASP7 regulation and exploring the potential of leveraging sites like CASP7 D79/K80 for developing selective caspase inhibitors. Moreover, the study demonstrated the utility of high-throughput screening techniques, such as the droplet microfluidic platform, in uncovering functional differences and aiding in targeted drug design for various peptide targets.
Caspases continue to be captivating subjects of research due to their essential roles in diverse biological processes. The recent study exploring the functional differences between caspase variants CASP3 and CASP7 provides valuable insights into their structure, function, and potential therapeutic applications. The utilization of high-throughput screening techniques and microfluidic platforms has advanced our understanding of these enzymes, opening up new possibilities for targeted drug design and the development of novel therapeutics.
“We hope that as more demonstrations of our platform’s utility follow, other researchers or private industries will begin to create easy-to-use prefabricated microfluidic chips and platforms that decrease barriers to entry “, the authors concluded.
Figures and the abstract are reproduced from Roychowdury, H., Romero, P.A. Microfluidic deep mutational scanning of the human executioner caspases reveals differences in structure and regulation. Cell Death Discov. 8, 7 (2022). https://doi.org/10.1038/s41420-021-00799-0 under Creative Commons Attribution 4.0 International License
Read the original article: Microfluidic deep mutational scanning of the human executioner caspases reveals differences in structure and regulation


