15 Sep High-throughput optimization of cyanobacterial cultivation using droplet-based microfluidics
Abstract
“Cyanobacteria are fast-growing, genetically accessible, photoautotrophs. Therefore, they have attracted interest as sustainable production platforms. However, the lack of techniques to systematically optimize cultivation parameters in a high-throughput manner is holding back progress towards industrialization. To overcome this bottleneck, here we introduce a droplet-based microfluidic platform capable of one- (1D) and two-dimension (2D) screening of key parameters in cyanobacterial cultivation. We successfully grew three different unicellular, biotechnologically relevant, cyanobacteria: Synechocystis sp. PCC 6803, Synechococcus elongatus UTEX 2973 and Synechococcus sp. UTEX 3154. This was followed by a highly-resolved 1D screening of nitrate, phosphate, carbonate, and salt concentrations. The 1D screening results suggested that nitrate and/or phosphate may be limiting nutrients in standard cultivation media. Finally, we use 2D screening to determine the optimal N:P ratio of BG-11. Application of the improved medium composition in a high-density cultivation setup led to an increase in biomass yield of up to 15.7%. This study demonstrates that droplet-based microfluidics can decrease the volume required for cyanobacterial cultivation and screening up to a thousand times while significantly increasing the multiplexing capacity. Going forward, microfluidics have the potential to play a significant role in the industrial exploitation of cyanobacteria.”

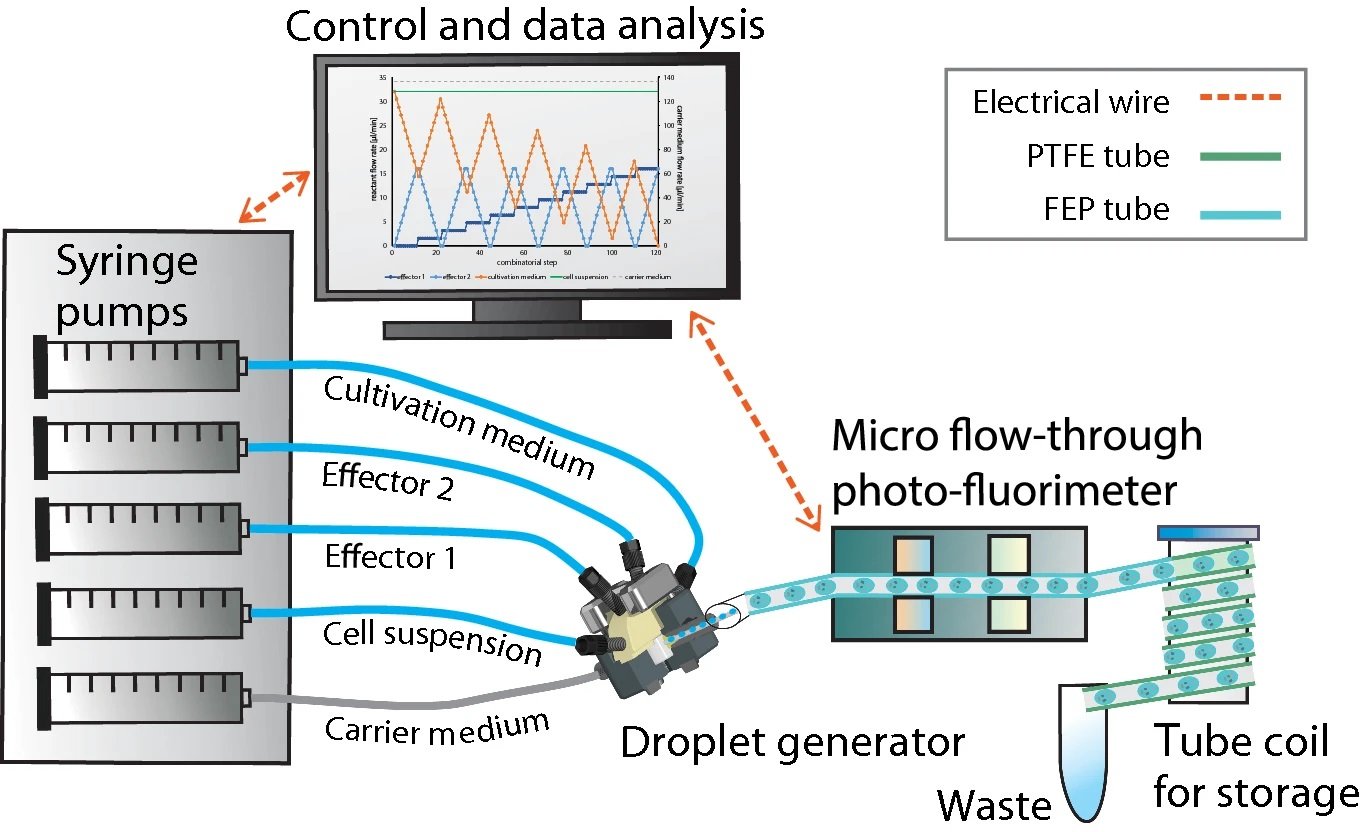
“Experimental droplet-based microfluidic setup for 1D and 2D screening of cultivation parameters used in this study. The illustration on the left shows a 5-channel syringe pump and the droplet generator which generates the droplets. The individual aqueous cell medium droplets are separated by the immiscible carrier medium. Droplet size/volume, spacing and composition can be adjusted via the controlled flow rate program of the syringe pump. The generated droplets are measured by a combined photo-fluorimetric micro flow-through detector unit. The droplet sequences are then collected and incubated in PTFE tube coils in an internally illuminated incubator.” Reproduced under Creative Commons Attribution 4.0 International License from Cao, J., Russo, D.A., Xie, T. et al. A droplet-based microfluidic platform enables high-throughput combinatorial optimization of cyanobacterial cultivation. Sci Rep 12, 15536 (2022).
Figures and the abstract are reproduced from Cao, J., Russo, D.A., Xie, T. et al. A droplet-based microfluidic platform enables high-throughput combinatorial optimization of cyanobacterial cultivation. Sci Rep 12, 15536 (2022). https://doi.org/10.1038/s41598-022-19773-6
Read the original article: A droplet-based microfluidic platform enables high-throughput combinatorial optimization of cyanobacterial cultivation


