24 Aug Microfluidic Control of Time-varying Stimuli Reveals Nuclear Remodeling in NF-κB Signaling
Understanding how cells decode signals from their environment is a central challenge in biology. One such pathway, NF-κB signaling, governs immune responses and inflammation. Traditional studies usually apply static doses of cytokines, but in living tissues, signals are anything but static—they fluctuate in strength and timing. The problem has been how to capture and study these dynamic cues in controlled conditions. As the authors explain,
“In this work, we investigate transmission of information about changing extracellular cytokine concentrations from receptor-level supramolecular assemblies of IKK kinases downstream to the NF-κB transcription factor. In a custom robot-controlled microfluidic cell culture, we simultaneously measure input-output encoding of IKK-NF-κB in dual fluorescent-reporter cells.”
Their solution is to use time as a control knob. With a microfluidics chip that precisely patterns cytokine pulses over cells, they compare single, finite pulses against dose-matched pulse trains. This custom microfluidic chip made it possible to observe, in real-time, how extracellular cytokine patterns are translated into nuclear NF-κB activity. The research team developed a robot-controlled microfluidic device that allowed precise delivery of time-varying cytokine pulses to single cells. This microfluidic chip made it possible to observe, in real-time, how extracellular cytokine patterns are translated into nuclear NF-κB activity.
The twist: keeping the total cytokine exposure the same, they show that certain pulse trains prolong IKK assemblies and cause NF-κB to linger in the nucleus, evidence of a mechanistic switch in nuclear export. The work reframes how microfluidic dosing can be used to probe signaling logic upstream of NF-κB and to study chromatin remodeling downstream.
Using CRISPR-engineered reporter cells, the team tracked upstream IKK activation and downstream NF-κB nuclear translocation simultaneously. When cells were exposed to single cytokine pulses, NF-κB responses followed expected rules: stronger or longer pulses produced greater nuclear activity. But under a series of shorter pulses, delivered via the microfluidic device, the dynamics shifted. Instead of resetting after each pulse, NF-κB remained in the nucleus for much longer than predicted, driven by prolonged IKK assemblies. This nuclear persistence pointed to chromatin remodeling, which the authors confirmed through mechanistic modeling. Essentially, repeated pulses induced a switch in nuclear export kinetics, locking NF-κB into an extended active state.
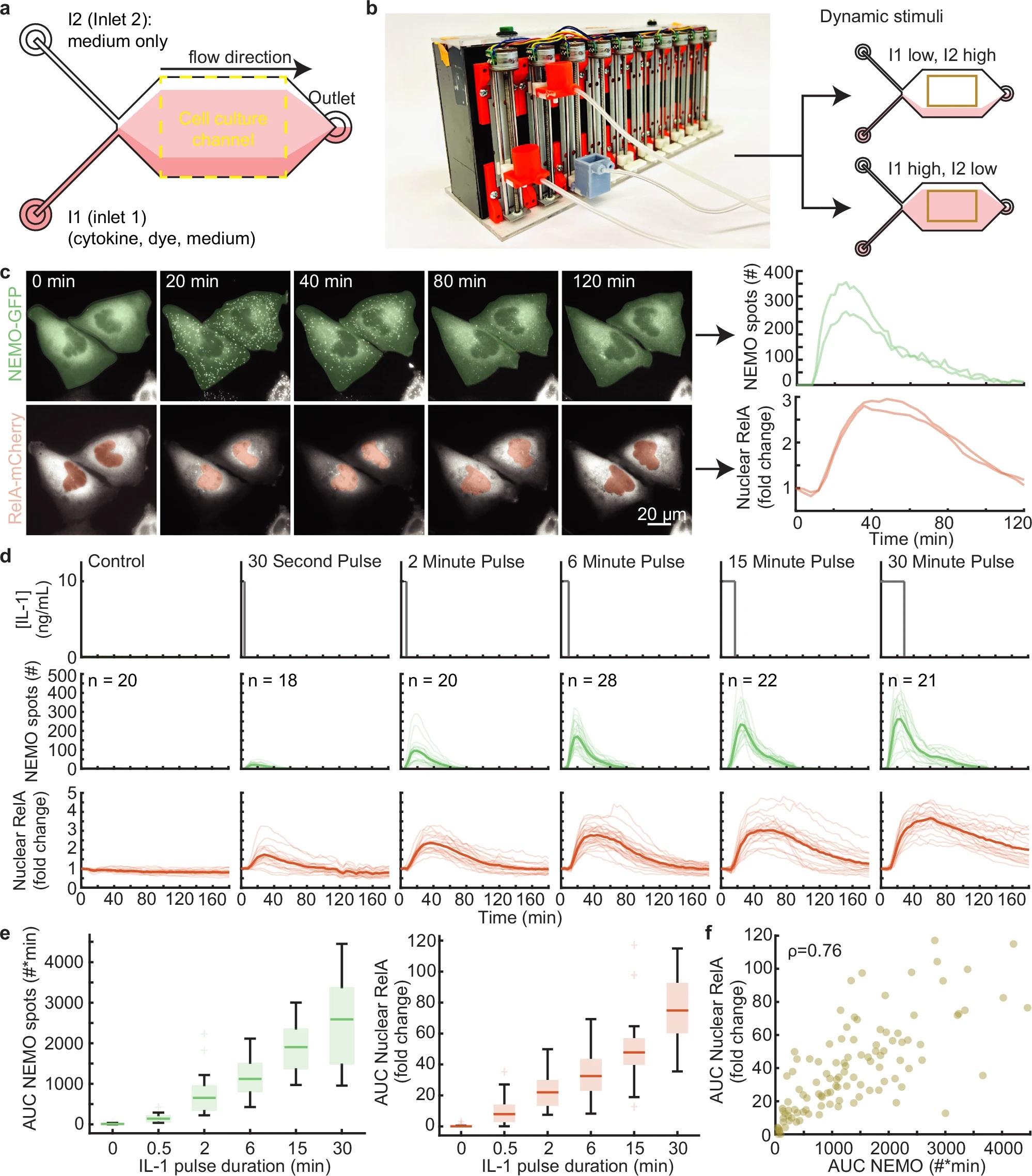
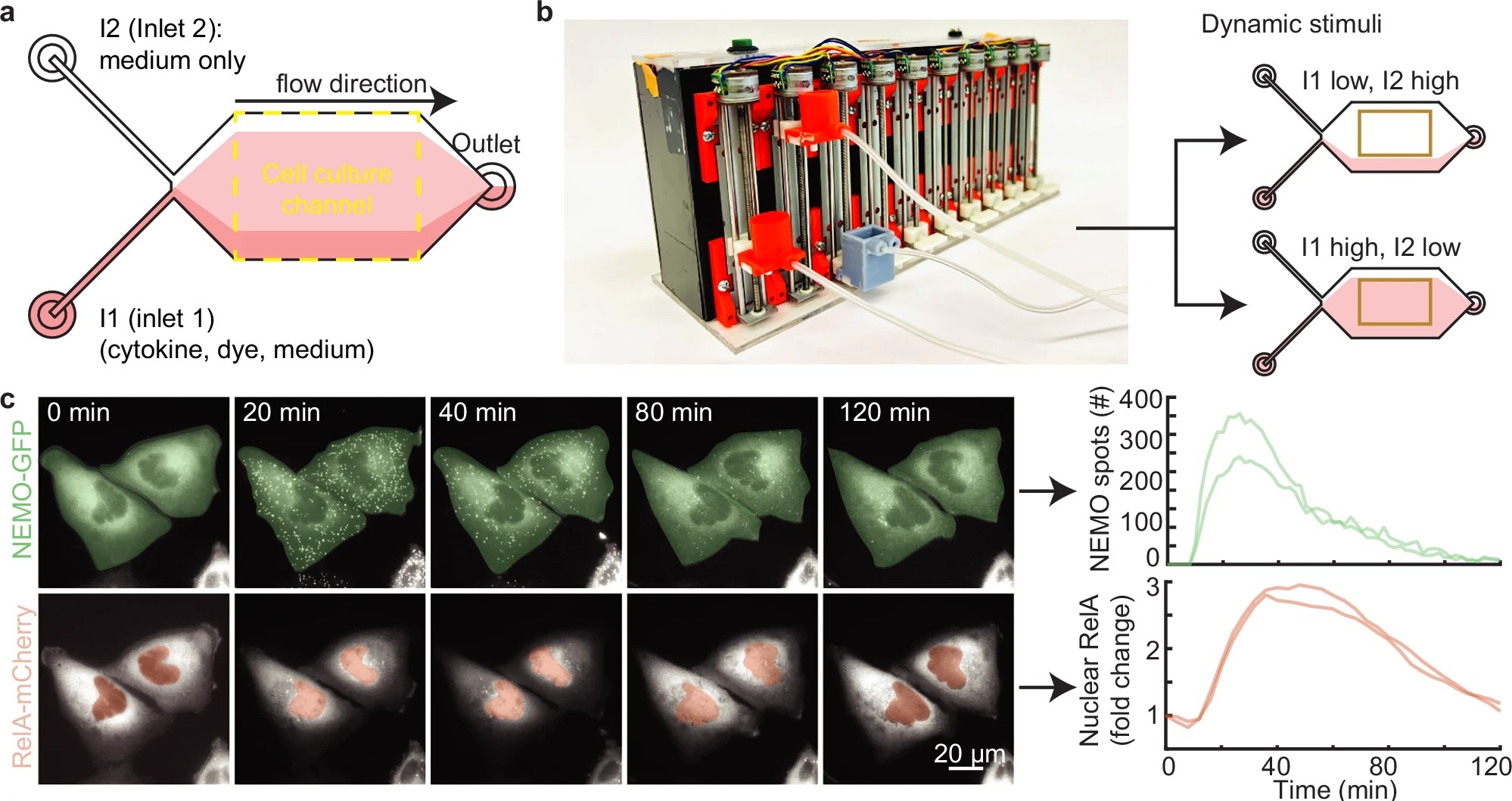
“a Schematic of a two-inlet, one outlet microfluidic chip with fluid flow from left to right. Inlet 1 (I1) contains a mixture of cytokine, dye, and medium, while inlet 2 (I2) contains only media. The fluorescent dye is used exclusively to establish the position of the cytokine-containing stream. U2OS cells are cultured and imaged within the central cell culture channel. On average, 22 cells were imaged for each condition. b Image of the custom gravity pump. Inlet reservoirs I1 and I2 are connected to the orange basins and the outlet connected to the gray basin. The gravity pump dynamically adjusts the relative heights of the basins to control fluid flow. When the I1 basin is positioned lower than I2, cells are exposed to media only (top) and when I1 is higher, cells are exposed to cytokine (bottom). c Time-lapse images of CRISPR-modified U2OS cells lines containing fluorescent protein fusions from their endogenous loci (left; see also Supplementary Movie 1). Quantification of upstream activated receptor complex measured through the formation and tracking of single EGFP-NEMO puncta (top). Quantification of downstream activation of the NF-κB (RelA) transcriptional system calculated by the fold change of mCh-RelA fluorescence in the nucleus (bottom). d Schematic illustrating the IL-1 stimulation profile applied to cells using the microfluidic device (top). Single cell time-courses for EGFP-NEMO spot numbers (green, middle) and mCh-RelA nuclear fold change (red, bottom) in response to a single pulse of IL-1 at 10 ng/ml with varying pulse durations. Light-colored trajectories represent individual cells, while bold trajectories represent the average response. e The area under the curve (AUC) for the number of IL-1 induced spots (green, left) and the fold change of nuclear RelA (red, right) increases with pulse duration. Single cell n values are listed in panel (d). f Same-cell correlations from dual-reporter cells show the aggregate AUC of activated receptor complexes and downstream transcriptional activity display a monotonic relationship for cells exposed to IL-1 pulses (Spearman ρ = 0.76). The unstimulated cells were not included in the correlation and plot. The scatterplot has n = 109 same-cell data points. For boxplots, the boxes represent the interquartile range with the median indicated as the center line. The bounds of the box correspond to the 25th and 75th percentiles. Whiskers extend to the minima and maxima values within 1.5 interquartile range, and points beyond are shown as outliers.” Reproduced from Smeal, S.W., Mokashi, C.S., Kim, A.H. et al. Time-varying stimuli that prolong IKK activation promote nuclear remodeling and mechanistic switching of NF-κB dynamics. Nat Commun 16, 7329 (2025). under Attribution 4.0 International License.
Their results demonstrated that temporal patterns of stimulation—not just concentration—determine how NF-κB operates. Importantly, the microfabricated system provided the necessary precision to reveal these hidden regulatory modes. The work highlights how microfluidic devices and microfabrication techniques can be leveraged to dissect complex cell signaling dynamics that would otherwise remain obscured.
In conclusion, by combining microfluidics with live-cell imaging and computational modeling, the study showed that time-varying cytokine inputs can reprogram NF-κB signaling through nuclear remodeling. These findings open doors for better understanding inflammation and immune regulation, particularly in disease contexts where cytokine signaling is highly dynamic.
Figures are reproduced from Smeal, S.W., Mokashi, C.S., Kim, A.H. et al. Time-varying stimuli that prolong IKK activation promote nuclear remodeling and mechanistic switching of NF-κB dynamics. Nat Commun 16, 7329 (2025). https://doi.org/10.1038/s41467-025-62837-0 under Creative Commons Attribution 4.0 International License.
Read the original article: Time-varying stimuli that prolong IKK activation promote nuclear remodeling and mechanistic switching of NF-κB dynamics
For more insights into the world of microfluidics and its burgeoning applications in biomedical research, stay tuned to our blog and explore the limitless possibilities that this technology unfolds. If you need high quality microfluidics chip for your experiments, do not hesitate to contact us.


