17 Nov Multiplexed isothermal detection of respiratory viruses on an autonomously loaded microfluidic chip
The problem addressed in this study centers on the need for rapid and dependable point-of-care diagnostics that can distinguish between respiratory viruses that often present with overlapping symptoms. Standard laboratory nucleic acid tests provide high accuracy but require specialized equipment, multistep sample preparation, and trained personnel, which limits access in primary care and resource-limited settings. Antigen tests are easy to use but often lack the sensitivity needed for reliable diagnosis. The gap between accuracy and accessibility motivates the development of diagnostic tools that can deliver laboratory-like performance at the point of care without complex workflows.
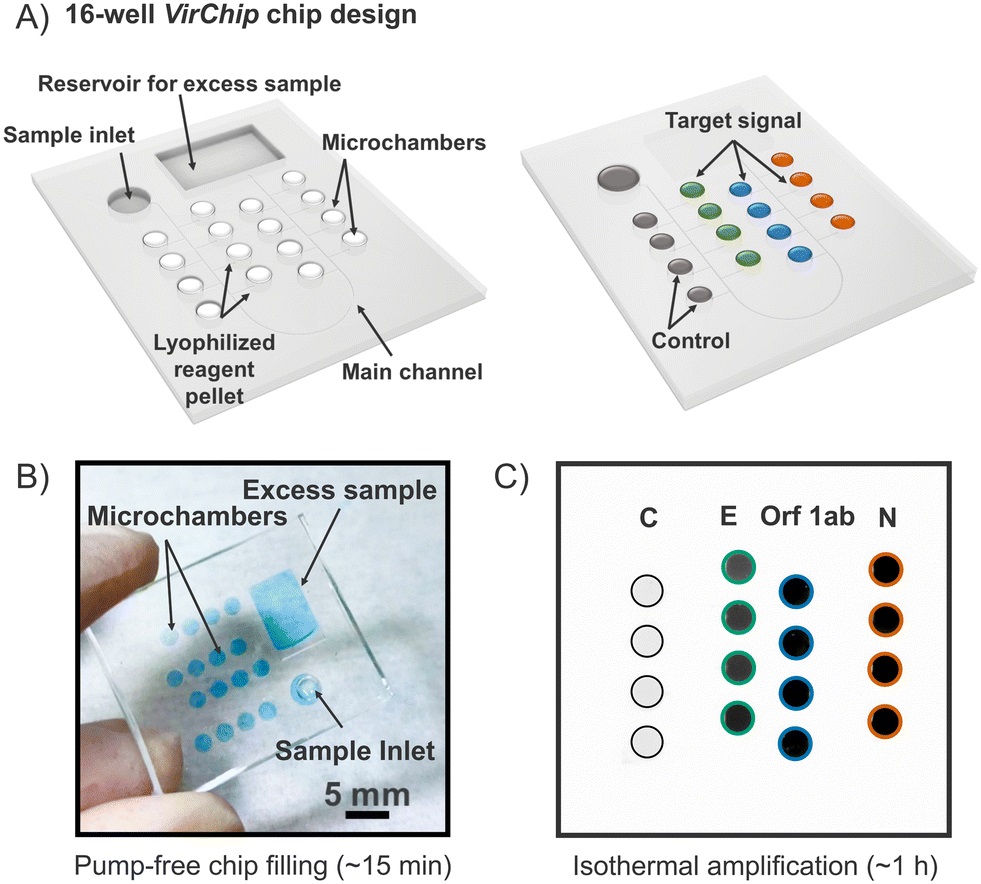
In an article published in Lab on a chip journal, the researchers propose a microfluidic chip called VirChip, which is a multiplexed, autonomously loaded microfluidic platform that performs isothermal amplification for simultaneous detection of several respiratory viral pathogens. The microfluidic device is designed to function without pumps or valves and is driven by a degas-based loading mechanism, which pulls the sample into the network of microchambers. The microfluidic platform incorporates primer-specific pellets that are freeze-dried directly inside the wells. Upon sample entry, the primers rehydrate and enable loop-mediated isothermal amplification of SARS-CoV-2, influenza A and B, and RSV A and B, all within a single microfluidic chip.

“(A) Schematic of VirChip platform (16-well) design, general operating and readout of amplified chips; (B) image of 16-well VirChip filled with a blue food dye solution (for demonstration only); (C) exemplary fluorescence image of a SARS-CoV-2 analysis with viral RNA isothermal amplification.” Reproduced from B. Berzina, K. Gupta, R. Suliman, P. Mirtschink, A. Dalpke, C. Werner, E. Krieg and L. D. Renner, Lab Chip, 2025, Advance Article , DOI:10.1039/D5LC00509D under Creative Commons Attribution 3.0 Unported Licence.
To create VirChip, the team microfabricated PDMS microfluidic devices using soft lithography and assembled multilayer chips containing either 16 or 24 wells. Each microwell was preloaded with primer mixes and stabilizing agents such as trehalose before freeze-drying. Following plasma bonding and vacuum degassing, the chips became ready for autonomous loading. When a clinical or spiked sample was pipetted into the inlet, the pressure differential rapidly filled the channels and wells. Mineral oil was added to protect each reaction chamber, and the chip was incubated at 65 °C to support isothermal amplification. Fluorescent dyes compatible with LAMP chemistry, particularly EvaGreen, allowed visualization of successful amplification in target wells while minimizing nonspecific signal. The microfluidic platform accepted either purified RNA or crude nasal swab samples preserved in non-lytic transport media, bypassing traditional extraction steps.
The microfluidic workflow produced clear and selective fluorescence signals for each pathogen target. In the 16-well configuration, the assay consistently detected 100 copies per microliter for SARS-CoV-2 with minimal cross-reactivity when influenza primer sets were included as controls. In the expanded 24-well version, the chip successfully distinguished between SARS-CoV-2, influenza A, influenza B, RSV A, and RSV B from both purified RNA and crude clinical samples. Patient samples with high and moderate viral loads showed 4 to 8 fold fluorescence increases in target wells, and the results closely aligned with qPCR data provided by clinical laboratories. The assay correctly identified negative samples and showed stable performance across various viral variants. The platform also demonstrated that crude heat-inactivated samples could be loaded directly into the microfluidic device without enzymatic lysis, simplifying preparation even further.
In conclusion, VirChip demonstrates that multiplexed nucleic acid detection can be carried out in a compact, low-cost microfluidic chip that requires minimal operator input. By combining autonomous loading, freeze-dried primers, and LAMP chemistry, the platform supports parallel detection of clinically relevant respiratory viruses from crude samples. The microfluidic device can be adapted to include additional targets and may eventually be integrated with portable fluorescence readout units. These developments show the potential for accessible, rapid, and sensitive diagnostics that support patient care outside centralized laboratories.
“With the current setup, VirChip will allow for on-site (patient-visit) rapid parallel detection of viral and bacterial pathogens, as well as in emergency room settings, to dramatically reduce the detection time of currently available technologies and to quickly isolate contagious patients. This simple, robust, and easy-to-use platform should enable a swift integration and will therefore simplify the workflow of emergency rooms.”, the authors concluded.
Figures are reproduced from B. Berzina, K. Gupta, R. Suliman, P. Mirtschink, A. Dalpke, C. Werner, E. Krieg and L. D. Renner, Lab Chip, 2025, Advance Article , DOI: 10.1039/D5LC00509D Creative Commons Attribution 3.0 Unported Licence.
Read the original article: Multiplexed detection of respiratory viral pathogens by isothermal amplification on an autonomously loaded chip at the point-of-care
For more insights into the world of microfluidics and its burgeoning applications in biomedical research, stay tuned to our blog and explore the limitless possibilities that this technology unfolds. If you need high quality microfluidics chip for your experiments, do not hesitate to contact us.


